41 label the energy diagram for a two‑step reaction.
PDF AP CHEMISTRY 2013 SCORING GUIDELINES - College Board intermediates in the proposed mechanism. In parts (e) and (f), students constructed a reaction-energy profile illustrating a two-step, exothermic reaction, and labeled the E a for the first step. Sample: 5A Score: 8 This response addresses the question and earned 8 points. In part (a) the distinction is made between Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. Question Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step.
Potential Energy Diagrams - Kentchemistry.com The extra energy is released to the surroundings. Reactants --> Products + Energy Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
Label the energy diagram for a two‑step reaction.
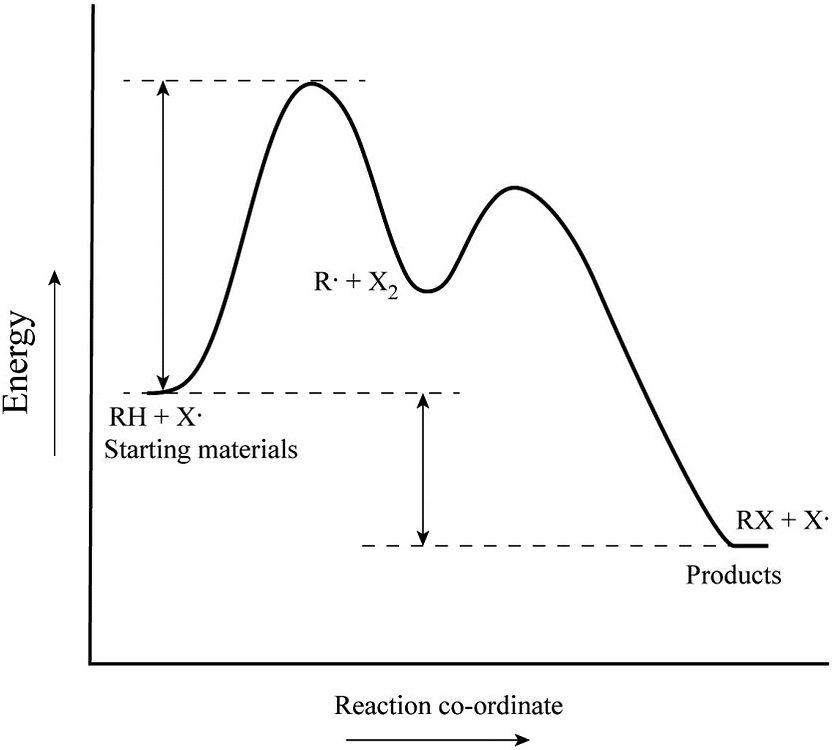
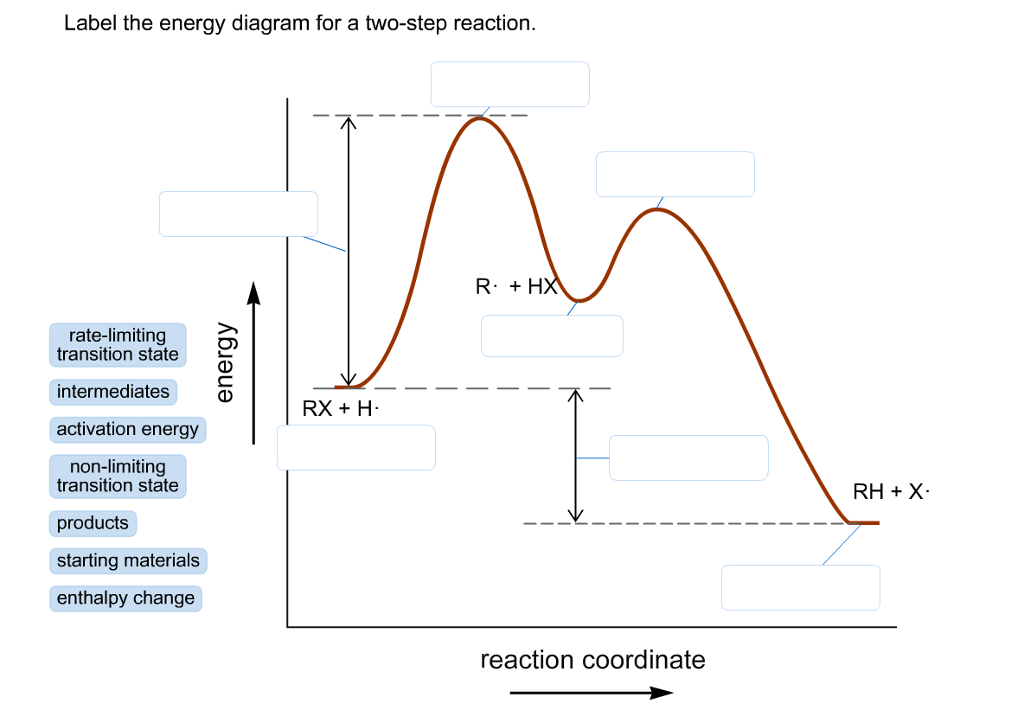
PDF Discussion Worksheet #6 Substitution Reactions Sn2 is a one step, 2 arrow mechanism with alkyl halides. Sn1 is a two step, 2 arrow mechanism (often followed by a deprotonation step) with alkyl halides. Problem 1: Label each as Sn1 or Sn2. Provide arrow mechanisms for each reaction, and draw an energy diagram assuming an overall exothermic process. Solved Label the energy diagram for a two-step reaction. R: | Chegg.com Label the energy diagram for a two-step reaction. R: +HX Energy M RX + H RH + X Reaction coordinate Answer Bank non-limiting transition state starting materials products enthalpy change rate-limiting transition state intermediates activation enco. Question: Label the energy diagram for a two-step reaction. R: +HX Energy M RX + H RH + X Reaction ... Biochem Ch. 20.1-20.2 Flashcards | Quizlet True. The active site of an enzyme. A.includes the entire enzyme. B.catalyzes the reaction. C.is converted to a product. D.increases the energy of reaction. E.is remote from the site of substrate attachment. B. Catalyzes the reaction. The formation of an enzyme-substrate complex is the __________ step in enzyme action.
Label the energy diagram for a two‑step reaction.. PDF Potential Energy Diagram Notes - ms. adrangi's teaching site Potential Energy DiagramsPotential Energy Diagrams o Generally, if a reaction requires a low activation energy, it is considered to be a spontaneous reaction (i.e. It is generally a fast reaction) • Reaction Mechanism o Elementary Process • A process in which a reaction occurs in one step (usually occurs between 2 particles) Ternary Process Answered: 11. Draw a potential energy diagram for… | bartleby Draw a potential energy diagram for a two-step reaction in which the activation energies are 10 kcal/mol (first step) and 3 kcal/mole (second step), the intermediate is 5 kcal/mol higher in energy than the reactants, and the products are 1 kcal/mol lower in energy than the reactants. [Solved] Please see an attachment for details | Course Hero Please see an attachment for details. Image transcription text. 11. Draw a potential energy diagram for a two-step reaction in which the activation. energies are 10 kcal/mol (first step) and 3 kcal/mole (second step), the intermediate. is 5 kcal/mol higher in energy than the reactants, and the products are 1 ... Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. PDF Potential Energy Diagram Worksheet ANSWERS Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to ... Draw an energy diagram for a reaction. Label the axis, PE of reactants = 350 KJ/mol, Ea = 100 KJ/mol, PE of products = 250 KJ/mol. 7. Is the reaction in # 6 exothermic or endothermic? Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ... Mechanisms and Potential Energy Diagrams | Chemistry for Non-Majors ... Summary A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
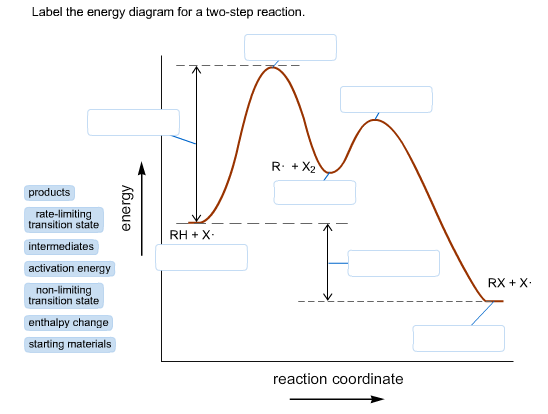
Question #62891 | Socratic The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ... Labeling an Energy Diagram Diagram - Quizlet Labeling an Energy Diagram STUDY Learn Flashcards Write Spell Test PLAY Match Gravity Created by Corey_WilliamsonPLUS Terms in this set (9) Reactants Starting ingredients for Forward reaction Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction Enthalpy (∆H) Answered: Label the components of an energy… | bartleby Label the components of an energy diagram for a spontaneous reaction. Question Transcribed Image Text: Label the components of an energy diagram for a spontaneous reaction. 4 Energy- Reaction progress- Answer Bank activation energy catalyzed reaction reactants products uncatalyzed reaction Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (49 ratings)
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Explanation Verified Reveal next step Reveal all steps Create a free account to see explanations
How to draw the potential energy diagram for this reaction? Since heat is released for C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction.
Answered: Sketch an energy diagram for a two-step… | bartleby Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram correspoding to reactant, product, intermediate, overall ΔG ‡, and overall ΔG°. Expert Solution Want to see the full answer? Check out a sample Q&A here
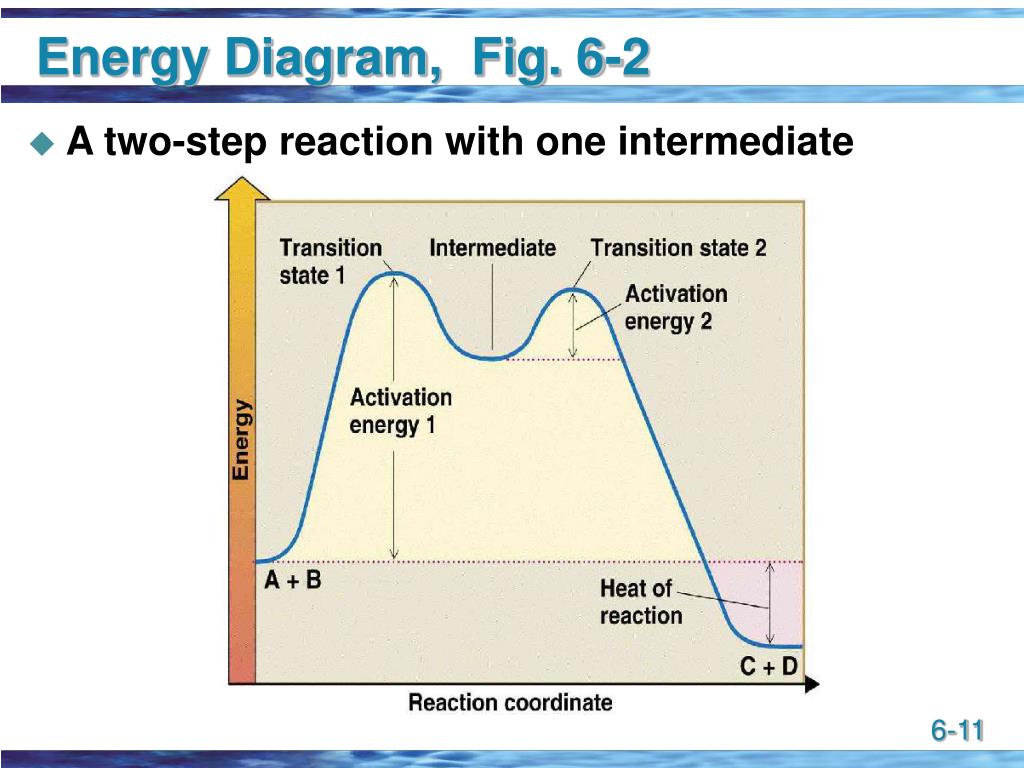
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Solved Label the energy diagram for a two-step reaction. - Chegg Expert Answer. 100% (190 ratings) Labelle …. View the full answer. Transcribed image text: Label the energy diagram for a two-step reaction.
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
Post a Comment for "41 label the energy diagram for a two‑step reaction."