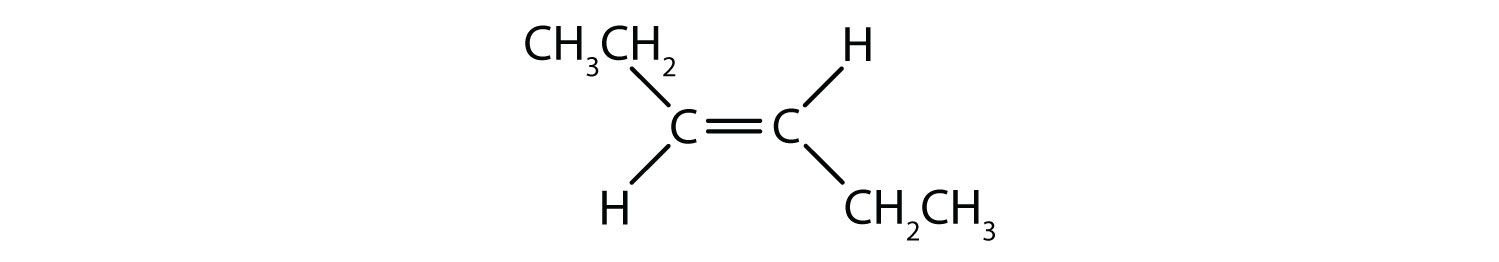
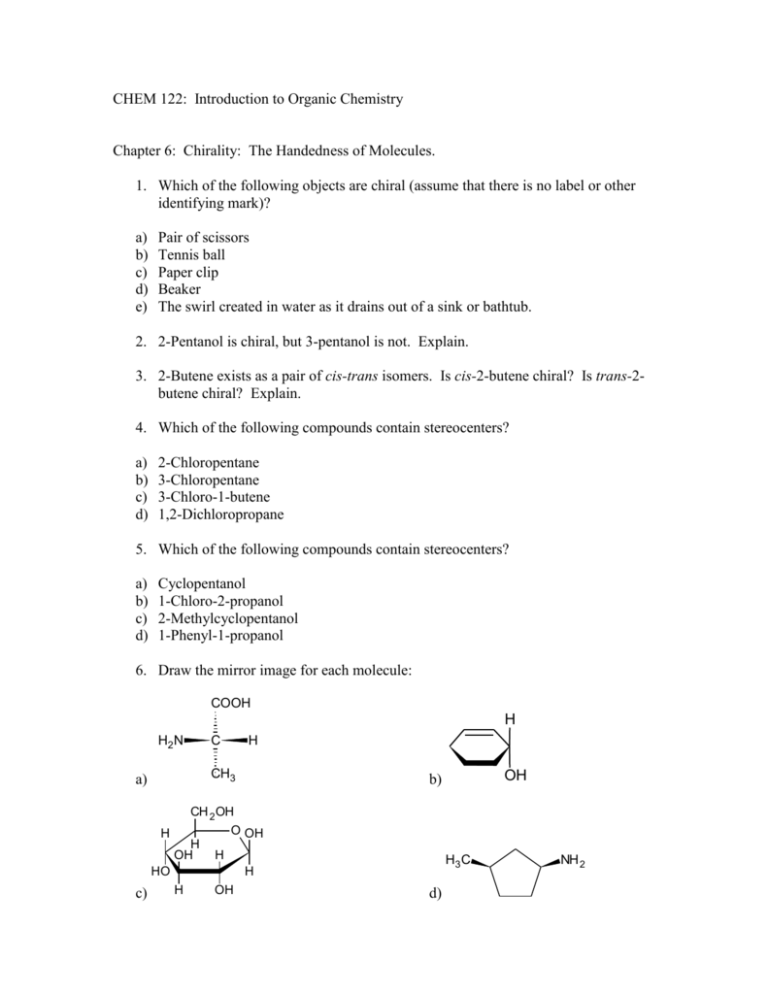
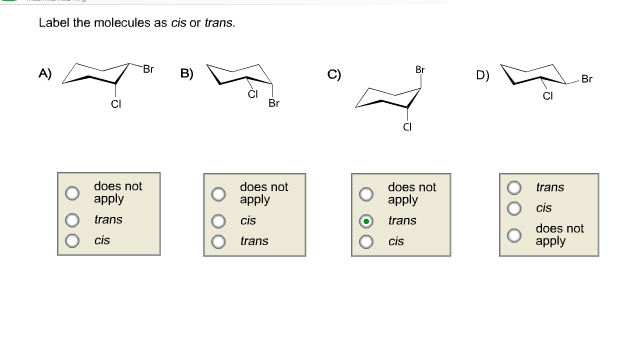
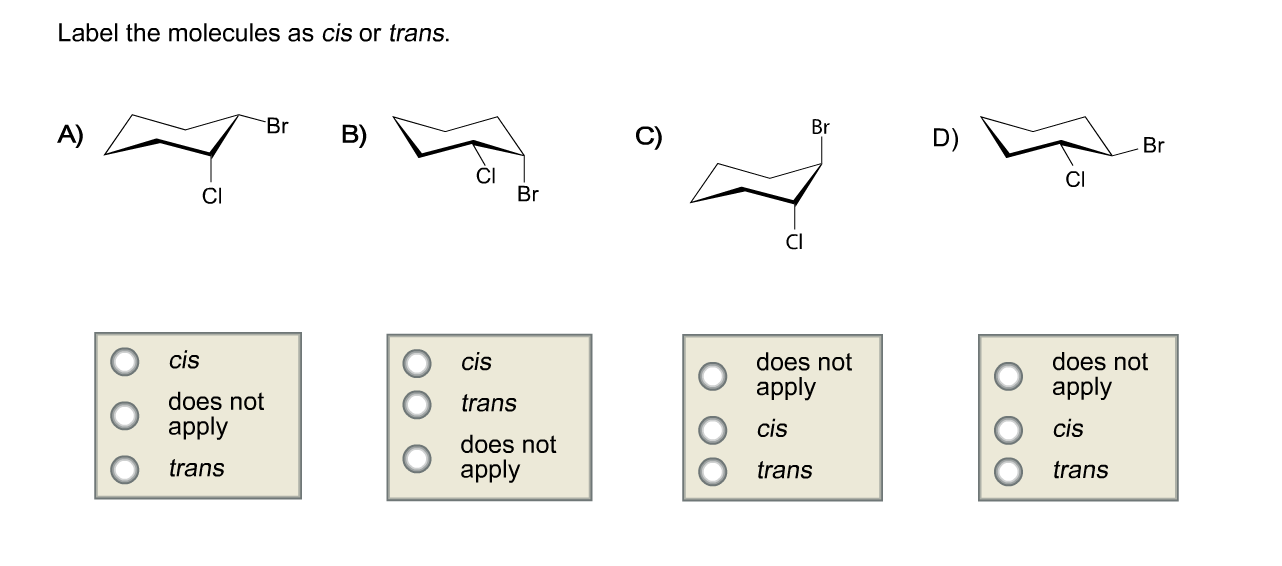
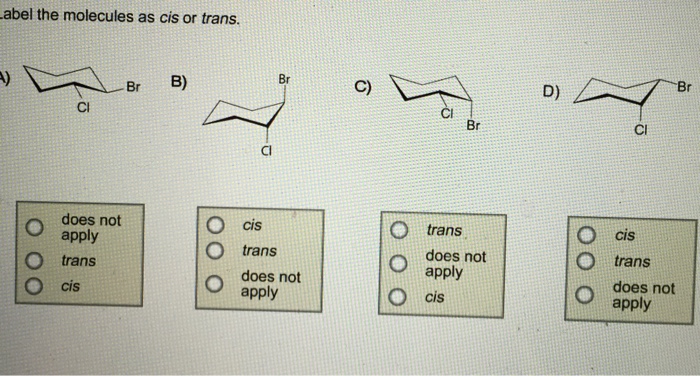
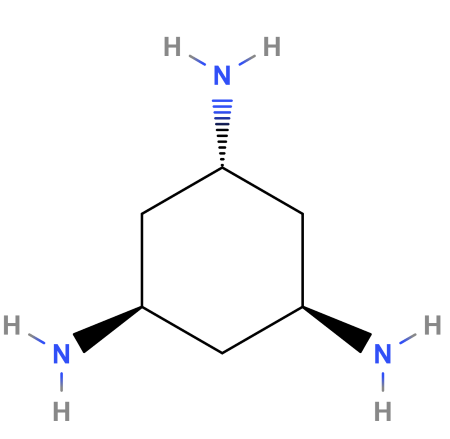
40 label the molecules as cis or trans.
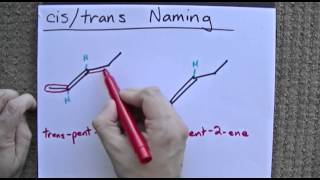
Organic Chemistry: Cis-Trans (Geometric) Isomerism But-2-ene satisfied the two criteria for cis-trans isomerism. As such, but-2-ene exists as two distinct and separate isomers. The isomer where the same groups are on the same side of the C=C double bond is known as the cis isomer.; The isomer where the same groups are on opposite side of the C=C double bond is known as the trans isomer.; Properties Study Chapter 4 Flashcards - Quizlet Label each pair of compounds below as: a. conformational isomers b. stereoisomers ... c. cis-trans isomers d. both b and c e. a, b and c ... a. identical molecules b. constitutional isomers c. stereoisomers d. different molecules. b. A: axial B: equatorial. Consider the two methyl groups indicated with letters in the following molecular model ...
What are Cis and Trans Double Bonds? That's Simple! If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image). The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures.
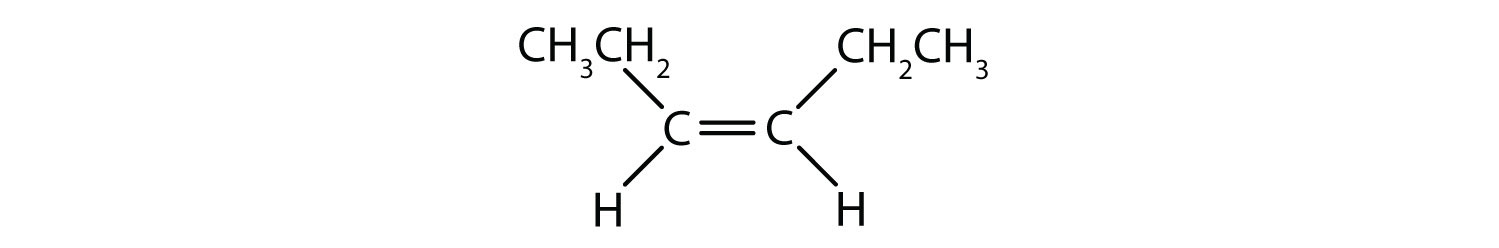
Label the molecules as cis or trans.
Rings: cis/trans and axial/equatorial relationships To show a specific isomer -- cis or trans -- we must somehow show how the two Cl atoms are oriented relative to the plane of the ring. cis -1,3-Dichlorocyclohexane The basic idea in both of these is that we can imagine the ring to be planar, and then show the groups above or below the plane of the ring. Althouse: Who started this use of "cis-" as the opposite of "trans ... 26.05.2022 · I really only came across cis and trans when I learned organic chemistry: When you have a long chain of single bonded carbon atoms, each of the carbon atoms will have two hydrogen atoms attached on opposite sides. If a double bond is formed between two of the carbons, the carbons will each have only one hydrogen attached. These hydrogens can be on … Difference Between Enantiomers and Diastereomers - VEDANTU Diastereomers are the stereoisomer compounds with molecules that do not mirror images of one another and that are not superimposable. The perfect example of diastereomers is when you look at the cis and trans isomer structures. (Image will be Uploaded Soon) See the cis-2-butene and the trans-2-butene structures below: (Image will be Uploaded Soon)
Label the molecules as cis or trans.. › 101 › cisWhat does cis mean? — TransHub Chemistry has used the terms trans and cis to talk about the arrangement of isomers, which are molecules or ions that have the same formula but different structures. They are used to describe the structure of a molecule, and whether an atom is on the same side as a similar atom, or a different side. Answered: Label the molecules as cis or trans. Br… | bartleby Solution for Label the molecules as cis or trans. Br Br A) B) D) Br Br CI does not apply does not аpply does not аply trans cis trans cis trans does not ...1 answer · Top answer: Step 1 Please find the file attac... chem.libretexts.org › Bookshelves › Physical_andInfrared Spectroscopy - Chemistry LibreTexts Apr 16, 2022 · The absorption of IR radiation by a molecule can be likened to two atoms attached to each other by a massless spring. Considering simple diatomic molecules, only one vibration is possible. The Hook's law potential on the other hand is based on an ideal spring \[\begin{align} F &= -kx \label{1} \\[4pt] &= -\dfrac{dV(x)}{dx} \label{2} \end{align}\] Kinome-wide polypharmacology profiling of small molecules by … 12.05.2022 · The organic molecules without biological activity records or clear chemical structures (SMILES string) and the inorganic compounds were removed. For each molecule, additional salts and solvents in the structure were removed using the Python script from Merget et al. 18. (2) High-confidence biochemical assays were kept (confidence level ≥ 8, ensuring that …
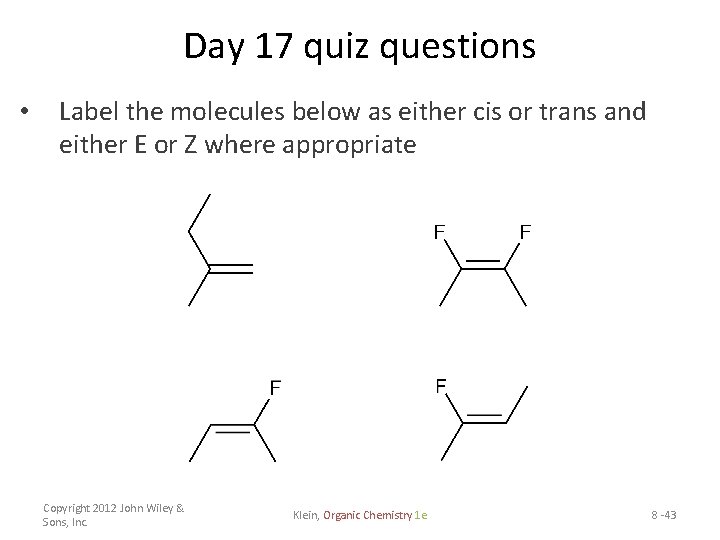
OneClass: Which of the following molecules can exist as cis and trans ... 14. Which of the following molecules have NO cis/trans isomers? If there is a cis/trans isomer, draw and label both isomers as E and Z. 15. Give the mechanism for the following reaction and label the rate limiting step + H20 heat 16. Predict the major product of both reactions and label products as Hoffman or Zaitsev's product. Constructing Z-Matrices | Gaussian.com 05.01.2017 · Last updated on: 05 January 2017. [G16 Rev. C.01] Quick Links. Basis Sets; Density Functional (DFT) Methods; Solvents List SCRF geometric (cis / trans) isomerism - chemguide Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. This page explains what stereoisomers are and how you recognise the possibility of geometric isomers in a molecule. Further down the page, you will find a link to a second page which describes the E-Z notation for naming geometric isomers. Infrared Spectroscopy - Chemistry LibreTexts 16.04.2022 · This causes the atom not to be stationary and to fluctuate continuously. Vibrational motions are defined by stretching and bending modes. These movements are easily defined for diatomic or triatomic molecules. This is not the case for large molecules due to several vibrational motions and interactions that will be experienced. When there is a ...
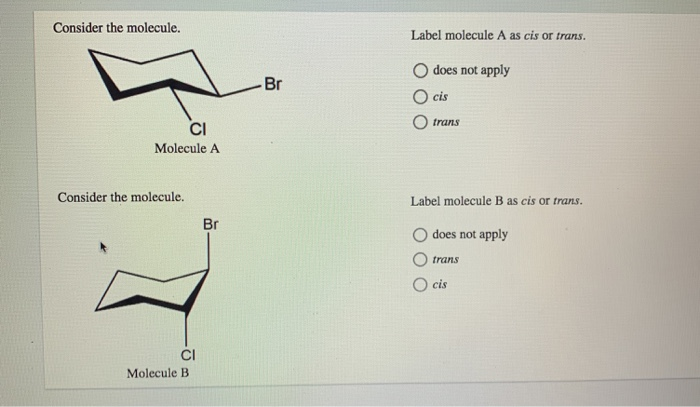
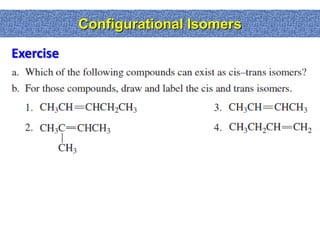
Label the above given disubstitued ring as cis or trans and as (a, a ... Label the above given disubstitued ring as cis or trans and as (a, ... List 2(p) Identical(q) Enantiomers(r)Diastereomers(s) Isomers(t) Different Molecules.1 answer · Top answer: Correct option is A) trans (e, e) Cis-trans and E-Z Isomerism : Pick your side | Stereochemistry If the two are on the same side, it's cis. If the two are on opposite sides, it's trans. It's that simple. An easy way to remember this convention is to look at the third letter of the word, for CI S the S stands for S ame and for TR A NS the A stands for A lternate. For example, let's look at 1,2-dichlorocyclohexane. Cis Trans and E Z Geometric Isomers When there is only one pi bond, you don't have to specify which carbon is cis or trans since. It's self-understood. When you have more than one double bond on the molecule, you must specify which is cis and which is trans. Take this molecule for example: 2,5-octadiene This molecule has 2 pi bonds. One cis and one trans. SOLVED:a. Which of the following compounds can exist as cis-trans ... For those compounds that can exist as cis and trans isomers, draw and label the isomers. Answer (a) 1 and 3 can exits as cis-trans isomers. 2 and 4 cannot exist as cis and trans isomers because identical substituents are bonded to the same $\mathrm{sp}^{2}$ hybridized carbon. View Answer. Related Courses. Chemistry 102.
SOLVED:a. Which of the following compounds can exist as cis-trans ... So, when you're talking about CIS and trans, you're talking about the location of the hydrogen atoms. So, if you're hydrogen atoms are on the same side of the double bond, they're assists. If the hydrogen atoms are on the opposite side, they're trans. Thanks. Look at the 2nd 1. Okay.
althouse.blogspot.com › 2022 › 05Who started this use of "cis-" as the opposite of "trans ... May 26, 2022 · cis and trans are used in chemistry. They're used in geography. They're used to mark a physical location Using them with respect to human beings is entirely different. And it's entirely ideological. Because to say that there a "cis women" and "trans women" is to say that there are no real "women".
(Get Answer) - 1. For each molecule, indicate whether cis-trans isomers ... If they do, draw the two isomers and label them as cis and trans. 2. For each molecule, indicate whether cis-trans isomers exist. If they do, draw the two isomers and label them as cis and trans. 3. Assign an IUPAC name to each of the following molecules. Include the prefi x cis- or trans- when appropriate.
› pmc › articlesSmall molecules in targeted cancer therapy: advances ... May 31, 2021 · If EGFR C797S and T790M mutations occur in trans, the combination of first- and third-generation EGFR TKIs has been reported to be an effective treatment strategy. 94 If they occur in cis, the patients are resistant to all approved EGFR TKIs; this is also a focus for research on fourth-generation EGFR TKIs. 94 Jia et al. reported an allosteric ...
Cis and Trans Isomers - Chemistry Steps Cis and Trans Isomers You may also need to classify two molecules with a cis/trans double bond or a ring system. Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, cis and trans isomers are diastereomers.
E-Z notation for geometric isomerism - chemguide The problem with the cis-trans system for naming geometric isomers. ... although the cis-trans system will only work for very straightforward molecules. Questions to test your understanding. If this is the first set of questions you have done, please read the introductory page before you start. You will need to use the BACK BUTTON on your ...
Label the following distributed ring as cis or trans class 12 ... - Vedantu Hence, the disubstituted ring is labeled as trans (e, e). so, the correct option is (A). Note: To identify cis and trans isomers, if two substituent groups are attached on the same side of the double bond then it is known as cis isomer and if two substituent groups are attached on the opposite side of the double bond then it is known as trans ...
› pmc › articlesTrans fats: What physicians should know - PMC Trans fats are unsaturated fats with trans double bonds instead of cis bonds. The type of bond affects the shape of the fatty acid chain. A trans bond creates a straight chain, whereas a cis bond results in a chain that is bent. Trans fats may be monounsaturated or polyunsaturated. The production of trans fats is a result of partial hydrogenation.
Small molecules in targeted cancer therapy: advances, challenges, … 31.05.2021 · If EGFR C797S and T790M mutations occur in trans, the combination of first- and third-generation EGFR TKIs has been reported to be an effective treatment strategy. 94 If they occur in cis, the patients are resistant to all approved EGFR TKIs; this is also a focus for research on fourth-generation EGFR TKIs. 94 Jia et al. reported an allosteric inhibitor EAI045 that …
Answered: Label the molecules as cis or trans. Br… | bartleby Label the molecules as cis or trans. Br B) CI 2 Br 6 Br D) -0 Br Question Transcribed Image Text:Label the molecules as cis or trans. Br B) CI does not apply trans cis Br trans cis does not apply 6 Br cis does not apply trans D) -G Br does not apply trans cis Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution
cis- and trans-alkenes - Faculty of Science 1,2-disubstituted alkenes are described as: cis - if the two alkyl groups, R-, are on the same side of the C=C. trans - if the two alkyl groups, R-, are on opposite sides of the C=C. these terms are inserted into the name as prefixes. cis-. trans-. For example, but-2-ene, where both R = methyl :
Solved Label the molecules as cis or trans. A) B) C) D - Chegg Question: Label the molecules as cis or trans. A) B) C) D) cis does not apply trans cos trans does not apply does not apply cis trans does not apply cis trans.1 answer · Top answer: A) Here Br is at equatorial position Cl is at axial pos...
cis-trans and E-Z naming scheme for alkenes - Khan Academy And that's all you have to do. And in the next few videos, we'll do a bunch of examples here so we can really figure out whether to label something with either an (E) or a (Z). But I wanted expose you to the cis- and trans- naming scheme, because that's sometimes used for simpler molecules where you only have one functional group on each side.
Trans fats: What physicians should know - PMC Unsaturated fats are molecules of fat containing one or more double bonds between two atoms of carbon at specific positions in the chain. Unsaturated fats come in a ‘cis’ form and a ‘trans’ form, according to the arrangement of the carbon chains across one or more double bonds. Trans fats are unsaturated fats with trans double bonds instead of cis bonds. The type of bond …
en.wikipedia.org › wiki › Golgi_apparatusGolgi apparatus - Wikipedia This collection of cisternae is broken down into cis, medial, and trans compartments, making up two main networks: the cis Golgi network (CGN) and the trans Golgi network (TGN). The CGN is the first cisternal structure, and the TGN is the final, from which proteins are packaged into vesicles destined to lysosomes , secretory vesicles, or the ...
Geometric Isomerism Cis- and Trans- Mean in Chemistry In geometrical isomer nomenclature, the prefix cis- and trans- are used to identify which side of the double bond the similar atoms are found. The cis- prefix is from the Latin meaning "on this side". In this case, the chlorine atoms are on the same side of the carbon-carbon double bond. This isomer is called cis-1,2-dichloroethene.
Golgi apparatus - Wikipedia The vesicles that leave the rough endoplasmic reticulum are transported to the cis face of the Golgi apparatus, where they fuse with the Golgi membrane and empty their contents into the lumen.Once inside the lumen, the molecules are modified, then sorted for transport to their next destinations. Those proteins destined for areas of the cell other than either the endoplasmic …
What does cis mean? — TransHub You may have heard the term cis being used before, maybe even being used to describe you, and wonder what it meant, or why the word would need to exist at all.. Cis, short for cisgender (pronounced sis-gender, or just sis), is a term that means whatever gender you are now is the same as what was presumed for you at birth.This simply means that when a parent or doctor …
Cis-Trans Isomers - Definition, Detailed Explanation with Examples - BYJUS Cis-trans isomers exhibit a type of stereoisomerism where the atoms have different spatial arrangements in three-dimensional space. In the field of organic chemistry, cis isomers contain functional groups on the same side of the carbon chain whereas the functional groups are on opposite sides in trans isomers.. This type of isomerism can arise in both organic and inorganic molecules.
Cis and Trans Isomers and Cis Trans Practice Problems - Chemistry Steps The cis and trans designation is included in the nomenclature of alkenes to distinguish the stereochemistry. The cis and trans designation is not determined based on alkyl groups only. For example, 2-pentene is not a symmetrical molecule thus we cannot have two identical alkyl groups on both carbons of the c=c bond:
Solved Label the molecules as cis or trans. | Chegg.com Label the molecules as cis or trans. Question: Label the molecules as cis or trans. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your ...
en.wikipedia.org › wiki › Trans_fatTrans fat - Wikipedia Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial processing of vegetable and fish oils in the early 20th century for use in margarine and later also in snack food, packaged baked goods, and for frying fast ...
Are cis and trans the same or different molecules? - Quora Cis and trans are fairly generic terms for relationships around some central bond or bonds that can't rotate: In 1,2-dimethylcyclopentane, cis and trans isomers are possible because the ring keeps the single bond from rotating. An open chain (e.g. butane) can't have cis or trans isomers because the central bond can rotate freely.
Trans fat - Wikipedia Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial processing of vegetable and fish oils in the early 20th century for use in margarine and later also in snack food, packaged baked goods, and for …
Cis-Trans Isomers (Geometric Isomers) - Lardbucket.org The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer. An isomer in which two substituent groups are attached to opposite sides of a double bond or ring in a molecule. (Latin trans, meaning "across") and is named trans -1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers)
7.5: Cis-Trans Isomerism in Alkenes - Chemistry LibreTexts The isomer in which the two methyl (CH 3) groups lie on the same side of the molecule is called the cis isomer (Latin cis, meaning "on this side") and is named cis-2-butene. The isomer with the two (CH 3) groups on opposite sides of the molecule is the trans isomer (Latin trans, meaning "across") and is named trans -2-butene.
Solved Label the molecules as cis or trans. does not apply | Chegg.com Question: Label the molecules as cis or trans. does not apply trans cis trans cis does not apply does not apply trans cis trans cis does not apply. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer.
How can you identify cis and trans isomers? + Example Explanation: Both of the isomers have exactly the same atoms joined up in exactly the same order .It means that the Van der Waals dispersion forces between the molecules will be identical in both cases. The difference between the two is that the 1) Cis I somer is polar whereas the T rans I somer is non − polar.
Cis and Trans - Organic Chemistry | Socratic The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon, as in but-2-ene. Then the two identical methyl groups are either cis or trans to each other, and the two identical hydrogen atoms are either cis or trans to each other.
Difference Between Enantiomers and Diastereomers - VEDANTU Diastereomers are the stereoisomer compounds with molecules that do not mirror images of one another and that are not superimposable. The perfect example of diastereomers is when you look at the cis and trans isomer structures. (Image will be Uploaded Soon) See the cis-2-butene and the trans-2-butene structures below: (Image will be Uploaded Soon)
Althouse: Who started this use of "cis-" as the opposite of "trans ... 26.05.2022 · I really only came across cis and trans when I learned organic chemistry: When you have a long chain of single bonded carbon atoms, each of the carbon atoms will have two hydrogen atoms attached on opposite sides. If a double bond is formed between two of the carbons, the carbons will each have only one hydrogen attached. These hydrogens can be on …
Rings: cis/trans and axial/equatorial relationships To show a specific isomer -- cis or trans -- we must somehow show how the two Cl atoms are oriented relative to the plane of the ring. cis -1,3-Dichlorocyclohexane The basic idea in both of these is that we can imagine the ring to be planar, and then show the groups above or below the plane of the ring.















Post a Comment for "40 label the molecules as cis or trans."