43 hybridization of so2
SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo... Hybridization of SO2 (Sulphur Dioxide) - CoolGyan.Org Free PDF download of JEE for Hybridization of SO2 (Sulphur Dioxide) to score more marks in exams, prepared by expert Subject teachers from the latest edition of CBSE books. Score high with CoolGyan and secure top rank in your exams.
SO2 Molecular Geometry, Hybridization, Lewis Structure SO2 Hybridization. The hybridization of SO2 is Sp2. Now hybridization of SO2 can be discerned in two ways, one is the theory and the 2nd is straight applying the formula. I would advise understanding the theory first and then you can absolutely go for the formula.. A quick edge for you, when 1 s orbital unites with 2 p orbitals it derives in Sp2 hybridization having 3 …

Hybridization of so2
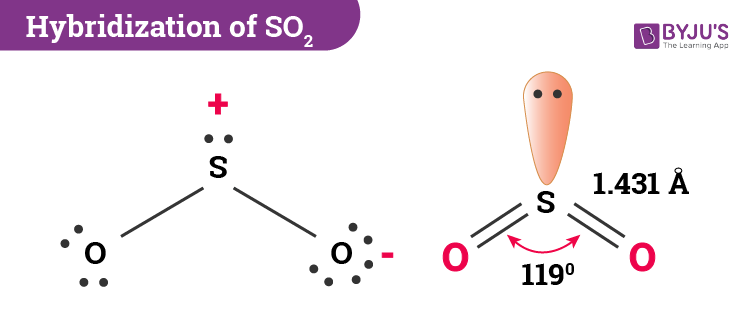
[Solved] Hybridisation in SO2 is: - Testbook SO 2 molecule, sulfur is the central atom. H = ½ (V + M - C + A) Putting this in the formula, we get H = 3 , which corresponds to sp2 hybridization. The structure of the SO2 molecule is. Thus, the hybridization in SO 2 is sp2. In SO 2, the lone pair of electrons is housed on an sp2 hybrid orbital. Due to this lone pair, there is electron-pair ... What Is SO2 Hybridization? - Reference.com SO2, commonly known as sulfur dioxide, has an sp3 hybridization. The molecular geometry of sulfur dioxide consists of two oxygen atoms bonded to the central sulfur atom. Hybridization explains the molecular structure of a compound. hybridization of so2 Hybridization explains the molecular structure of a compound. 4. ), The Secret Science of Solving Crossword Puzzles, Racist Phrases to Remove From Your Mental Lexicon. It has 5 things or regions around the central atom. p has 3 orbitals I just learned about hybridization today, and there are some cases that I really don't understand at all.
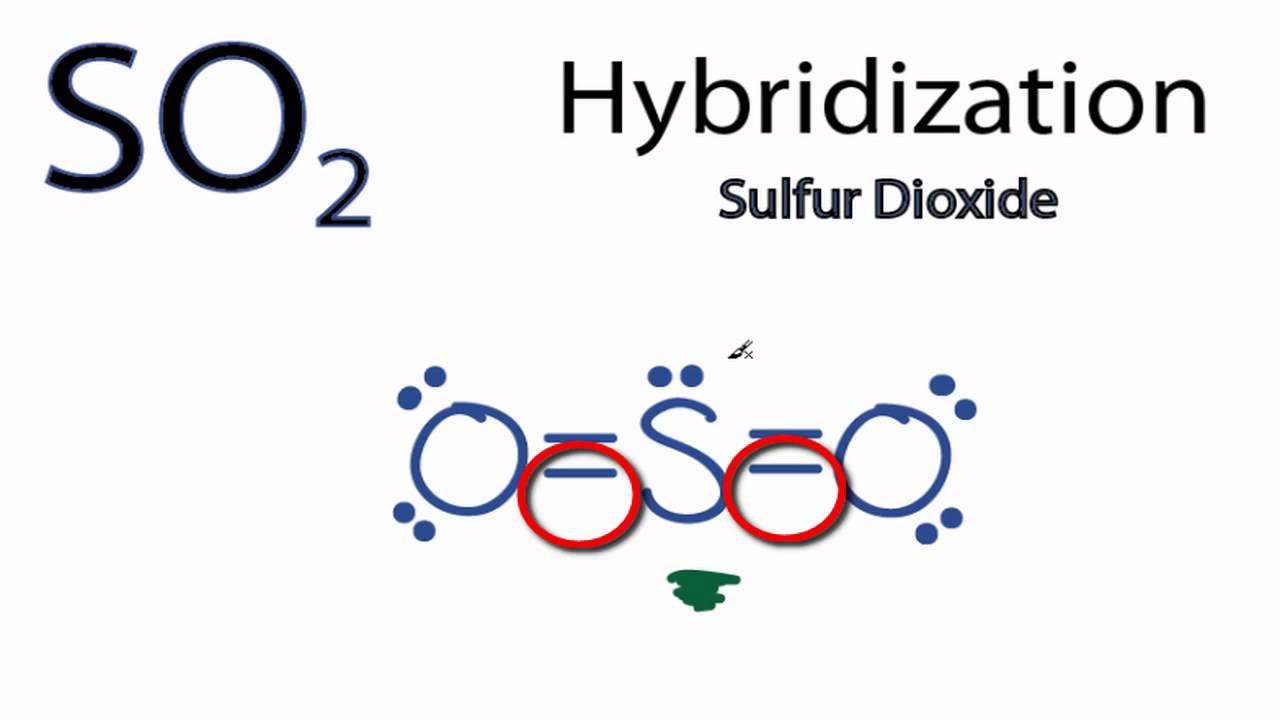
Hybridization of so2. SO2 Lewis Structure, Molecular Geometry, Hybridization, Polar or ... SO2 has an SP2 type hybridization. We can easily determine any of the molecule molecular orbital (i.e., hybridization) with the help of given formula which is, No. of hybridization = [V + X - C + A]/2 where, V = No. of valence electrons of central atom X = No. of monovalent atoms around the central atom C = Positive charge on cation Hybridisation in SO2 molecule is: - toppr.com >> Hybridization >> Hybridisation in SO2 molecule is: ... Verified by Toppr. Correct option is B) In Sulphur Dioxide, the S atom has 2 bond pairs and 1 lone pair, so it requires 3 hybrid orbitals. So the hybridisation is s p 2. Solve any question of Chemical Bonding and Molecular Structure with:- What is the hybridization of S in SO2? - Firstlawcomic In sulphur dioxide, the hybridization that takes place is sp2 type. To determine this, we will first look at the sulphur atom which will be the central atom. During the formation of SO2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O. Hybridization of NO2 - Introduction, Lewis Structure, Molecular ... The simple way to determine the hybridization of NO2 is by counting the bonds and lone electron pairs around the nitrogen atom and by drawing the Lewis structure. We will also find that in nitrogen dioxide, there are two sigma bonds and one lone electron pair. If we apply the hybridization rule now, then it states that if the sum of the number ...
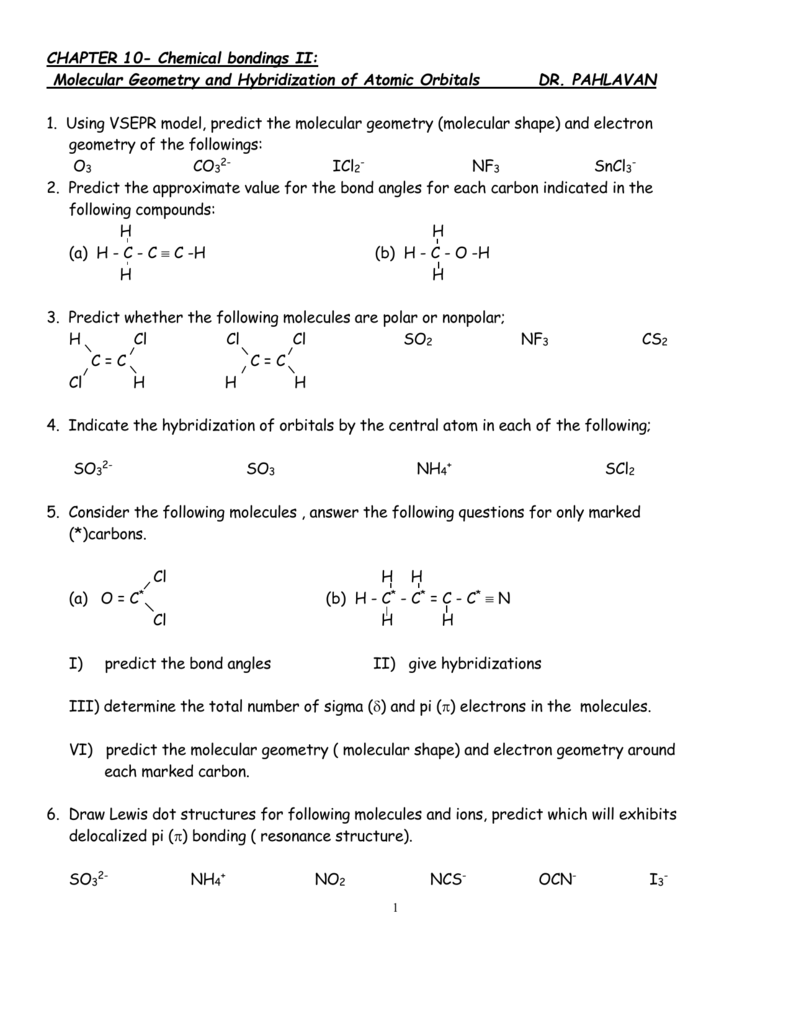
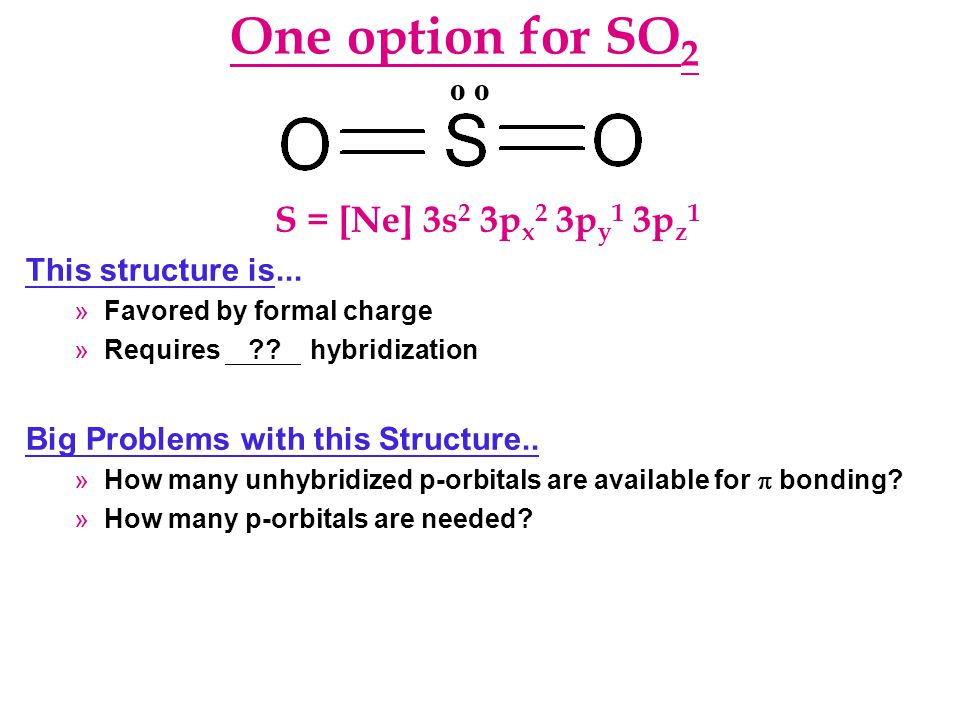
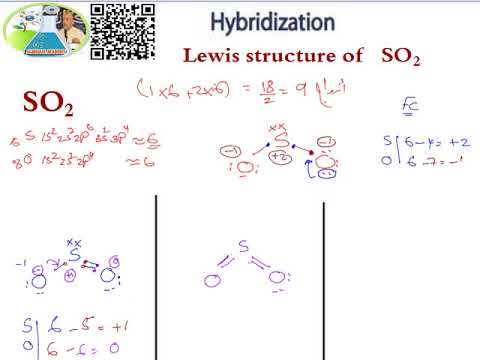
What is the hybridization of SO2? - Answers Of orbitals= 2+1=3 since needed no. Of orbitals is 3, there will beSP2 hybridization. Structure: Triangular bond angle= 120 degree ... What is the hybridization of SO2? Wiki User. ∙ 2012-12-10 ... What is the hybridization in "CO"_2? | Socratic Explanation: You must first draw the Lewis structure for CO2. According to VSEPR theory, we can use the steric number (SN) to determine the hybridization of an atom. SN = number of lone pairs + number of atoms directly attached to the atom. SN = 2 corresponds to sp hybridization. SN = 3 corresponds to sp2 hybridization. CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories Enter the email address you signed up with and we'll email you a reset link. Orbital Hybridization: sp1, sp2, and sp3 Hybridization Aug 01, 2021 · Hybridization and Shape of SO2. Step 1: Write the Lewis structure. Sulfur’s valency may be 2 or 4 or 6. Oxygen’s valency = one. Each oxygen makes two bonds with sulfur atom. One is sigma bond and the second one is pi bond. The total number of bonds formed by sulfur with two oxygen atoms = four.
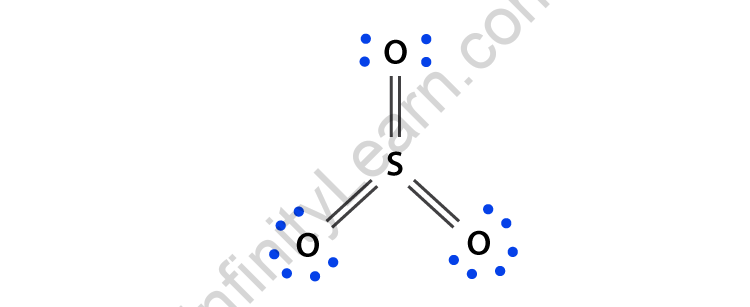
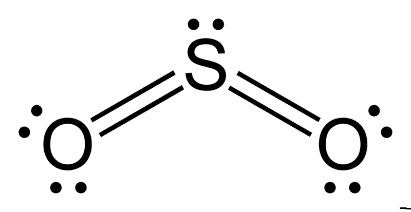
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry ... SO2 comprises a Sulfur atom surrounded by two oxygen atoms. In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom. The hybridization of the SO2 is given by sp2. SO2 has a Bent molecular structure and shape with bond angles of 120°. About Priyanka H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles Apr 05, 2021 · We have previously discussed the Lewis structures of CO2, O3, SO2, SO3 and more. Today we are going to learn about the Lewis structure of H2O molecule along with its molecular geometry and shape. ... H2O Hybridization. When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. These orbitals help us to predict ... Hybridization of sulfur in sulfur dioxide - ECHEMI One of the canonical structures for sulfur dioxide - $\ce{SO2}$ - has sulfur (with a lone electron pair) double bonded to each oxygen atom to form a total of 4 bonds for sulfur - which can be achieved via valence expansion into empty d-orbitals.What then is the hybridization of the valence-expanded sulfur?It is described as sp². But how can that be? solucionario quimica de raymond chang 12 edicion Enter the email address you signed up with and we'll email you a reset link.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular … Jan 27, 2022 · SO2 Hybridization. Sulfur Dioxide comprises a central Sulfur atom surrounded by Oxygen atoms on either side. An easy way to determine the hybridization of a compound is to determine the number of electron domains it possesses. There are two covalent bonds and one lone pair. There are three electron domains, and this gives SO 2 an sp 2 ...
SO2 Hybridization (Sulfur Dioxide) - YouTube Hello Everyone!Today in this video we are going to help you find out the hybridization of SO2 molecule. It is a chemical formula for Sulfur Dioxide. To find ...
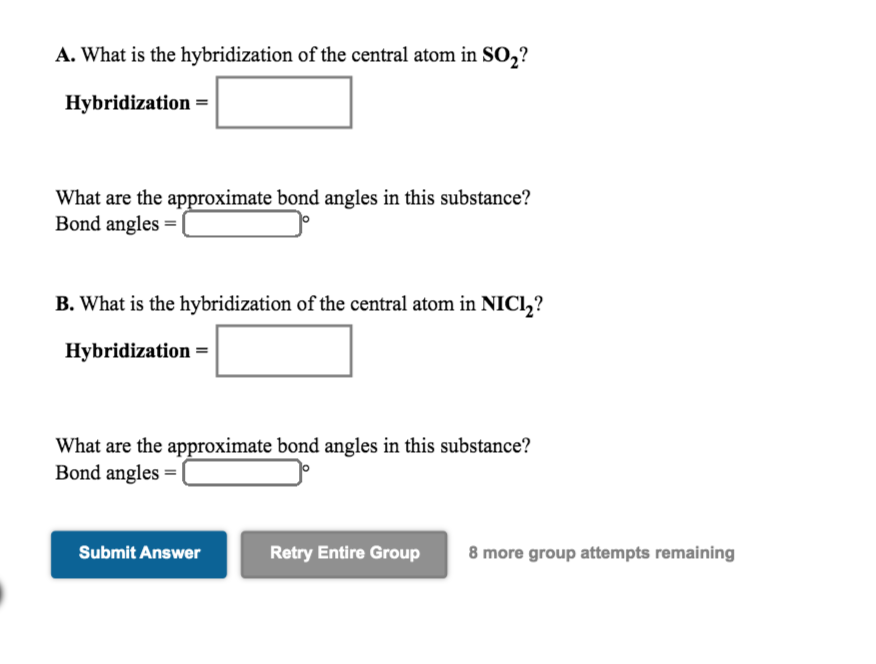
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram H = 3 = Sp2 hybridization. I hope the hybridization of SO2 is clear from both the explained concepts. SO2 Molecular geometry The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs.
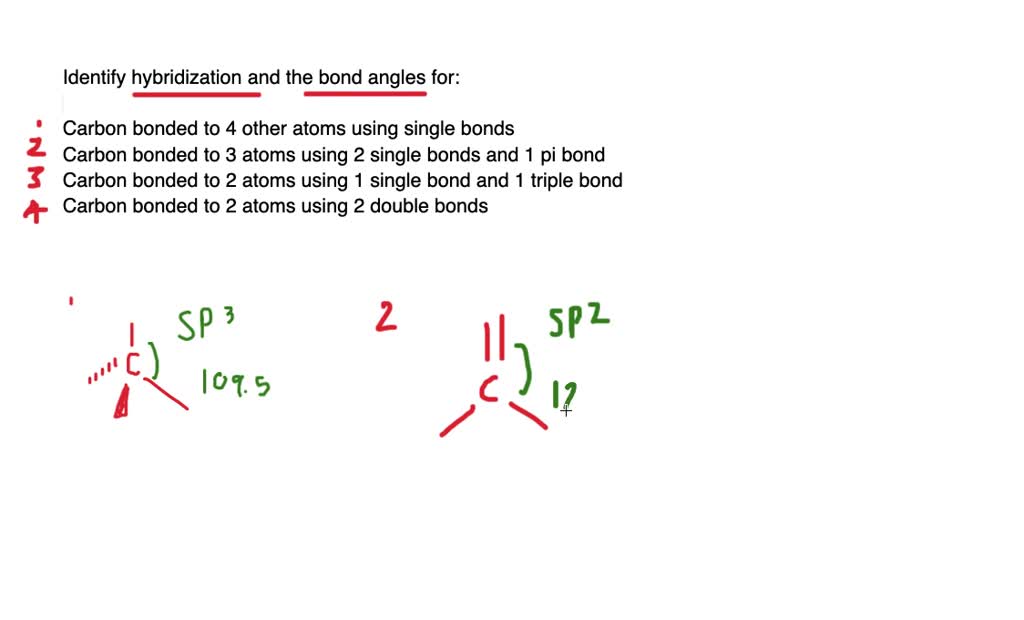
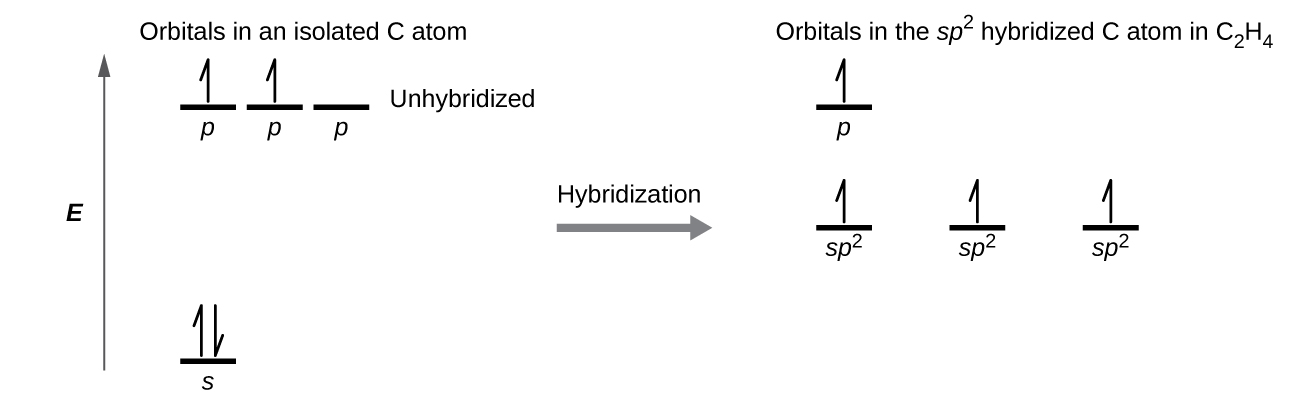
Hybridization of Carbon - Molecular Geometry and Bond Angles 1. sp Hybridization. Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. Example: Hybridization of CO 2. 2. sp 2 Hybridization
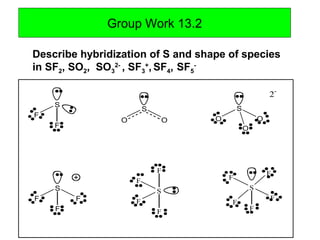
How is the hybridization of SO3 2-determined? - Quora Answer (1 of 4): 1. Draw the Lewis structure. 2. Count the domains around the S where you find electrons. A domain is a region in space where you find electrons. I see 4 here: a lone pair, two single bonds and a double bond. 3. You need the same number of hybrid orbitals as you have domains. In...
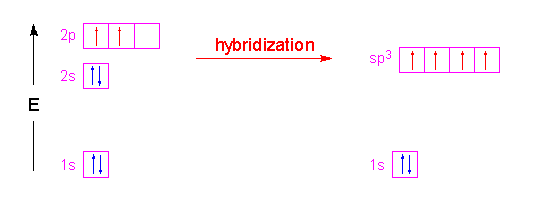
Hybridization of SO₂ - Definition, Electron Geometry Vs ... - VEDANTU The hybridization of the two oxygen atoms is sp2 as properly. SO2 is a bent shape (molecular geometry). Sulfur desires 6 electrons, and so does oxygen. Consequently, 6×three=18 valence electrons distribute in the course of the structure placing 4 for every of two double bonds makes use of up to eight.
SO2 Molecular Geometry, Hybridization, Lewis Structure & MO Diagram SO2 Hybridization Now coming to the formula part. The formula for finding hybridization of any compound is; H = ½ [ V+M-C+A] Where, H depicts Hybridization V is the no. of valence electrons M is the count of monovalent atoms present C represents the cationic charge A represents the anionic charge Here, if H is 2, it's Sp hybridization.
SO2 Lewis Structure, Hybridization, Molecular Geometry, and … Jul 24, 2022 · The hybridization of SO2 is Sp2. Now hybridization of SO2 can be understood in two ways, one is the theory and the 2nd is directly applying the formula. I would suggest learning the theory first and then you can surely go for the formal. A quick tip for you, when 1 s orbital unites with 2 p orbitals it results in Sp2 hybridization having 3 ...
Answered: raw the structure of BF4- Calculate… | bartleby A: Hybridization of the following molecules: PO43- = sp3 XeF2 = sp3d KrBrCl2F = sp3d2 SO2 = sp2 PCl5 =… Q: Predict the electron-domain geometry and hybridizationof the central atom in SO3? A: SO3 has Sulfur as the central atom and oxygen is the neighboring atom;
What is hybridization of SO2? - Firstlawcomic What is hybridization of SO2? In sulphur dioxide, the hybridization that takes place is sp2 type. To determine this, we will first look at the sulphur atom which will be the central atom. During the formation of SO2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O.
Hybridization of SO3 (Sulphur Trioxide): Hybridization of S in SO3 To understand the hybridization of sulphur trioxide we have to understand the bonding between sulphur and oxygen. If we draw and look at the Lewis structure sulphur will be the central atom and will have three double bonds with oxygen. In this, there is one sigma and one pi bond formed. This gives us the sp 2 hybridization type.
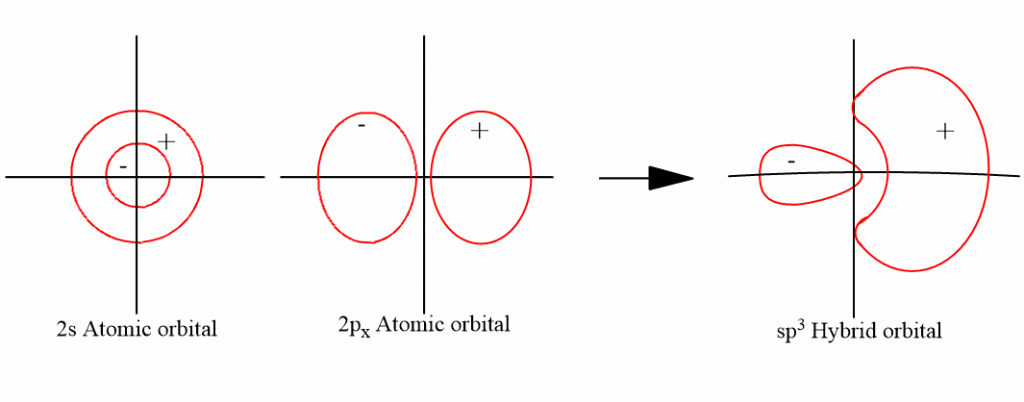
Hybridization of NH3 (Ammonia): Hybridization of Nitrogen in … In NH 3 hybridization, the three hydrogens will be based around the central atom of nitrogen. The hydrogen atoms are just s orbitals overlapping those sp 3 orbitals. NH 3 Molecular Geometry And Bond Angles. If we look at the molecular geometry of ammonia it has a trigonal pyramidal or distorted tetrahedral structure.
so2 hybridization explained - Get Education The hybridization of SO2 is Sp2. Now can determine the hybridization of SO2 in 2 ways, one is the theory, and the second is the straight applying the formula. I would undoubtedly suggest recognizing the concept first and afterwards. You can opt for the procedure.
SO2 Lewis Structure: Drawings,Hybridization,Shape,Charges,Pair And ... SO2 hybridization: The hybridization takes place in SO2 is sp2 type . For formation of SO2 ,we need 2 double bond outside the sulfur atom, while sulfur is in the middle or central. During formation of SO2 , the hybridization used is SP2. There are 2 sigma, 2 pi and one lone pair of electrons. The SO2 is in bent shape.
What is the Hybridization of Sulphur Dioxide? - BYJUS In SO 2 hybridization two 3p orbitals and one 3s orbital get hybridized. Two-hybrid orbitals contain unpaired electrons and one hybrid orbital will have the lone pair. The 3d and 3p orbitals remain the same, and they form pi bonds. SO 2 Molecular Geometry And Bond Angles SO 2 molecular geometry is considered to V-shaped or bent.
SO4 2- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry Hence SO42- ion has an sp3 hybridization. SO42- Molecular Geometry We can determine the molecular geometry of any given molecule using the VSEPR theory model and the AXN notation method. For example, for the Sulphate ion, the AXN notation would be AX4, as it forms bonds with four oxygen atoms.
hybridization of so2 Hybridization explains the molecular structure of a compound. 4. ), The Secret Science of Solving Crossword Puzzles, Racist Phrases to Remove From Your Mental Lexicon. It has 5 things or regions around the central atom. p has 3 orbitals I just learned about hybridization today, and there are some cases that I really don't understand at all.
What Is SO2 Hybridization? - Reference.com SO2, commonly known as sulfur dioxide, has an sp3 hybridization. The molecular geometry of sulfur dioxide consists of two oxygen atoms bonded to the central sulfur atom. Hybridization explains the molecular structure of a compound.
[Solved] Hybridisation in SO2 is: - Testbook SO 2 molecule, sulfur is the central atom. H = ½ (V + M - C + A) Putting this in the formula, we get H = 3 , which corresponds to sp2 hybridization. The structure of the SO2 molecule is. Thus, the hybridization in SO 2 is sp2. In SO 2, the lone pair of electrons is housed on an sp2 hybrid orbital. Due to this lone pair, there is electron-pair ...

























Post a Comment for "43 hybridization of so2"