39 phase diagram of pure solvent and solution
The important differences between the phase diagram of a pure solvent ... Science Chemistry Chemistry: The Molecular Nature of Matter and Change The important differences between the phase diagram of a pure solvent and that of the solution of that solvent are to be determined. Concept introduction: The phase diagram is a combination of liquid-gas, solid-liquid, and solid-gas curves and determines the effect of temperature and pressure on the phase of a substance. Liquid–liquid equilibrium measurements and computational study … 16.08.2022 · Many types of biotechnological interest molecules, such as proteins, peptides, and other tiny bioactive compounds, could be extracted and recovered using systems comprising two liquid phases at ...
Solvent engineering for high-performance inorganic–organic … Jul 06, 2014 · Figure 1 presents a schematic diagram of the cell architecture and deposition of perovskite materials by the solvent-engineering technology by means of spin-coating, as well as X-ray diffraction ...

Phase diagram of pure solvent and solution
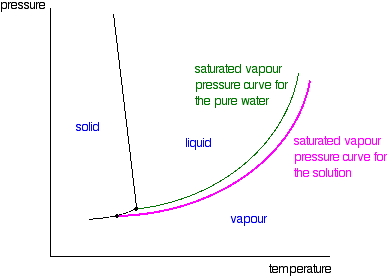
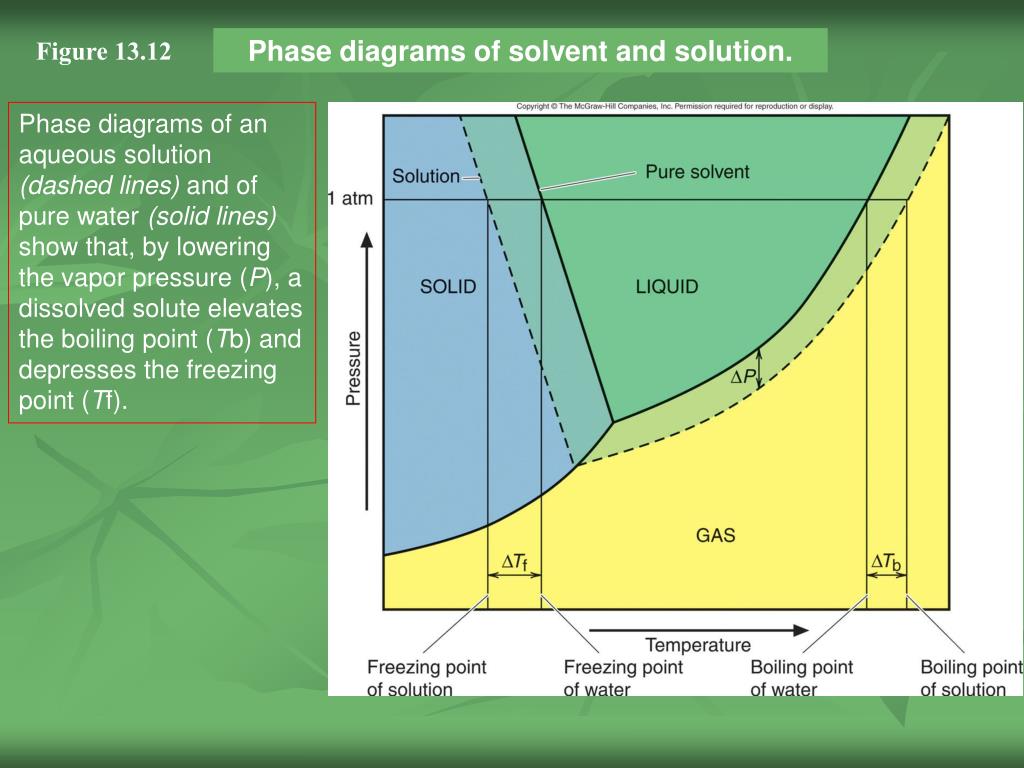
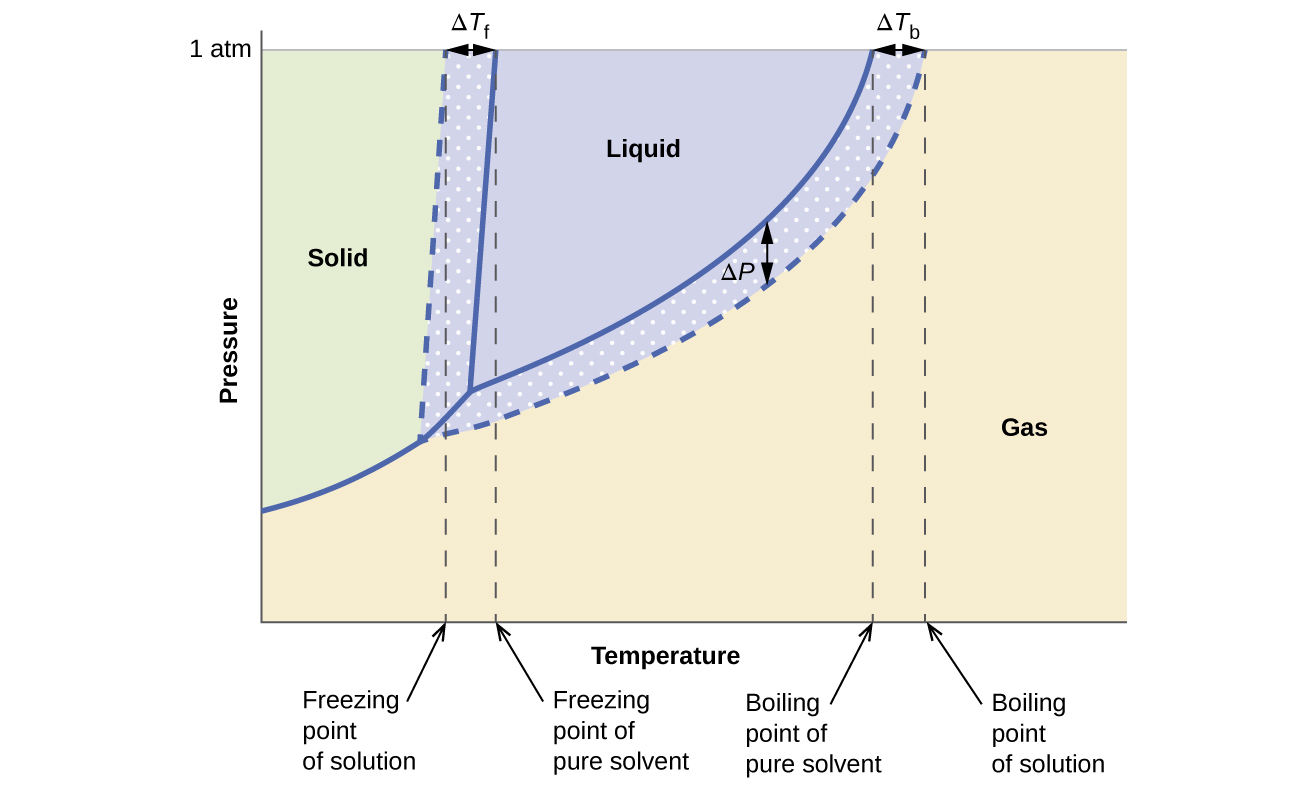
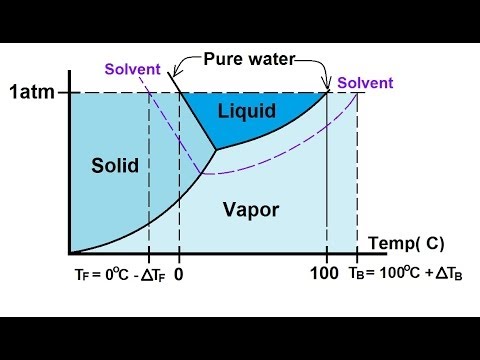
What are the most important differences between the phase di - Quizlet Two differences we can see on phase diagrams of a pure solvent and a solution are changes in phase transition temperatures \\textbf{phase transition temperatures} phase transition temperatures.Solution will always have a higher boiling point \\textbf{higher boiling point} higher boiling point and lower freezing point \\textbf{lower freezing point} lower freezing point (check pages 501, 502). PDF phase diagram solvent solution - Just Only phase diagram solvent solution Effect of Solute on Phase Diagram of Water SOLIDLIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTfΔTb ΔP Temperature Phase (matter) - Wikipedia In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform.: 86 : 3 Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, …
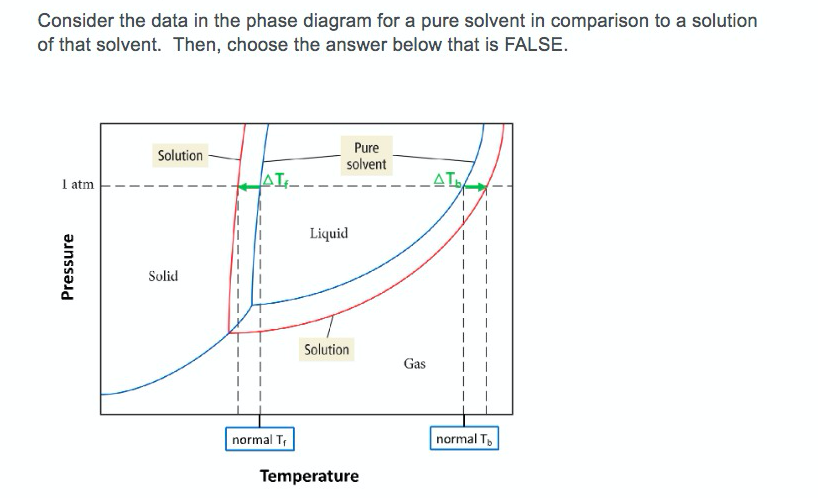
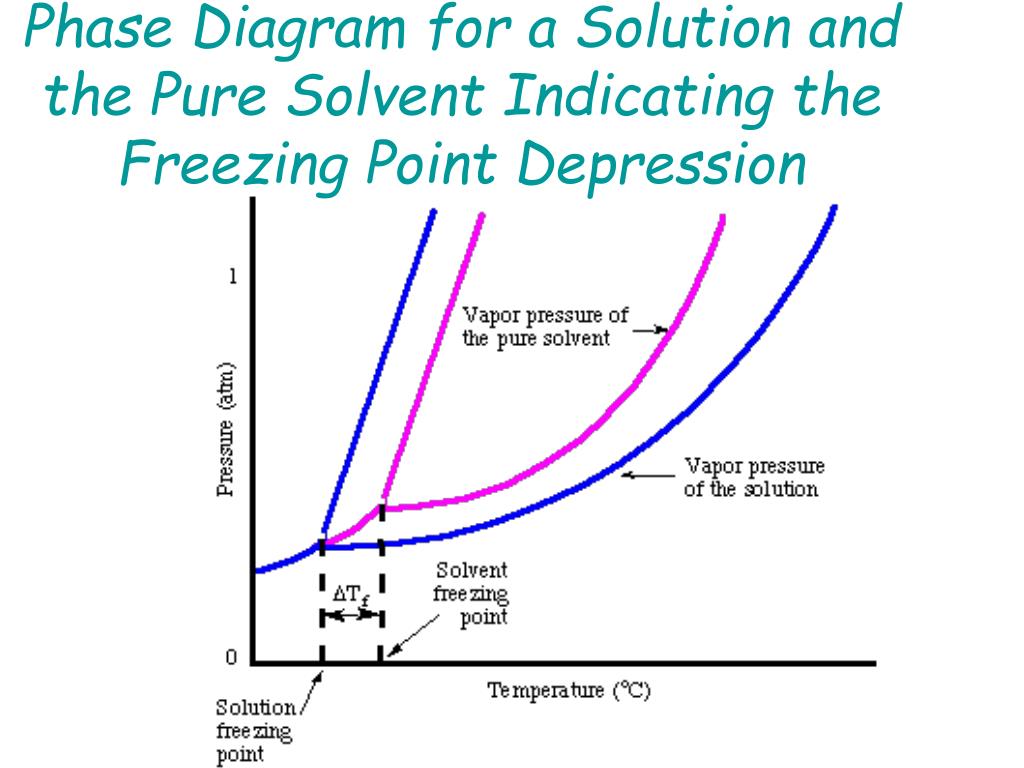
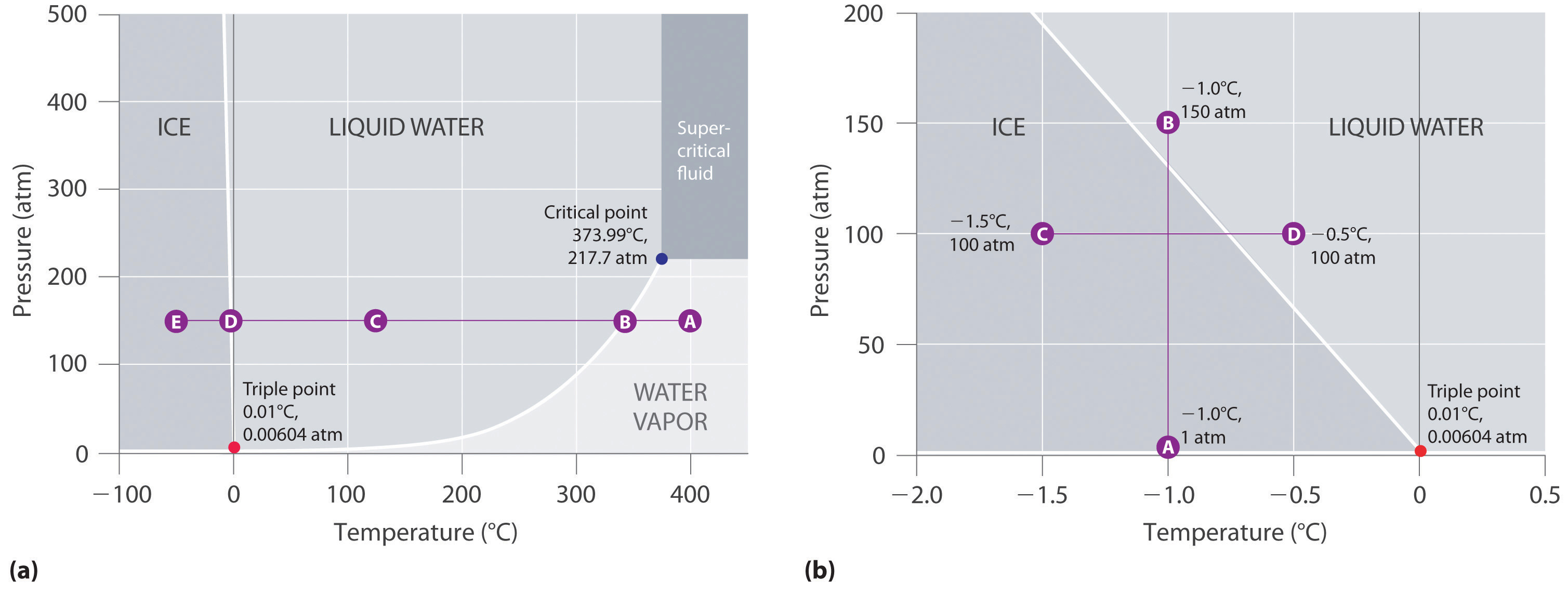
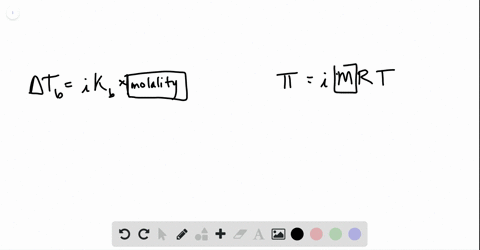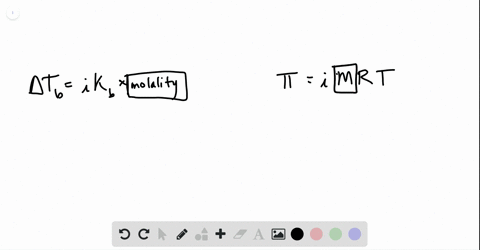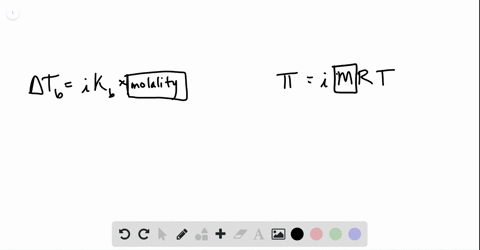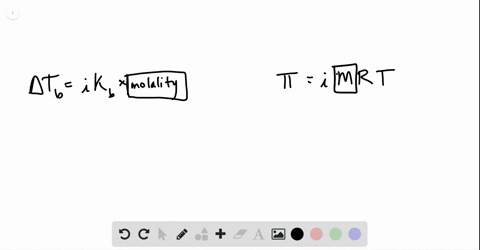
Phase diagram of pure solvent and solution. The figure shows two phase diagrams, one for a pure liquid (black line ... Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards. ΔT b = T b − T * b = iKbm Phase diagram - Wikipedia A phase diagram for a binary system displaying an eutectic point. One type of phase diagram plots temperature against the relative concentrations of two substances in a binary mixture called a binary phase diagram, as shown at right. Such a mixture can be either a solid solution, eutectic or peritectic, among others. (Get Answer) - Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature. Feb 24 2022 12:42 PM. Expert's Answer ... Solved The phase diagrams for a pure solvent and the solvent - Chegg Chemistry questions and answers. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that ...
Process design and phase diagrams : Phasediagram Phase diagrams for pure compounds are visual tools to display the properties of pure compounds forming various phases at different temperatures and pressures. In a phase diagram for a pure component, solid-liquid, vapor-liquid, solid-vapor, and solid-solid equilibria can be shown in the same diagram. This is not possible for a binary system. Phase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, … Mixed Solvent systems : Phasediagram In all the depicted phase diagrams, the markers represent experimental data while the solubility equilibrium lines were calculated with the Extended UNIQUAC model. Notice that the temperatures vary between the diagrams. In all the graphs below, the focus is on the lower part of the diagrams. The apex with pure K 2 SO 4 is therefore not shown. NCERT Exemplar Class 9 Science Chapter 2 Is Matter Around Us Pure 23.06.2022 · Solution: In smoke, the dispersed phase is solid and the dispersion medium is gas. In fog, the dispersed phase is liquid and the dispersion medium is gas. Question 18. Classify the following as physical or chemical properties. (a) The composition of a sample of steel is 98% iron, 1.5% carbon and 0.5% other elements. (b) Zinc dissolves in hydrochloric acid with the evolution …
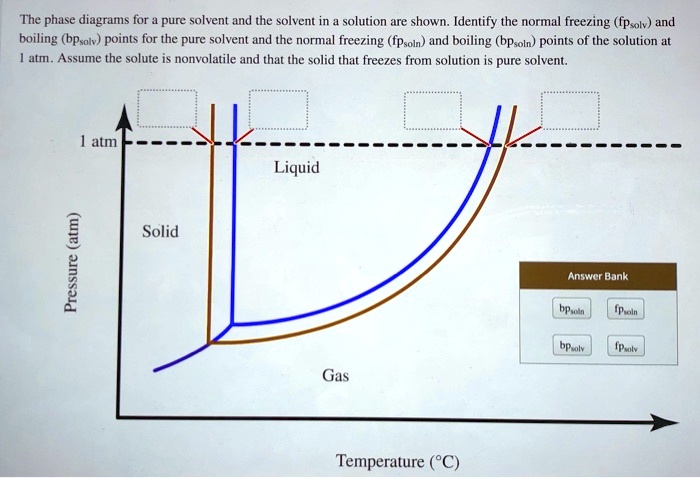
Answered: The phase diagrams for a pure solvent… | bartleby identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm liquid solid answer bank fpsolv bpsolv fpsoln bpsoln gas temperature … Draw a phase diagram showing how the phase boundaries differ for a pure ... The phase boundaries are shown for both solvent and solution. The liquid gas boundary for a solution is at a higher temperature than that for the pure solvent. This indicates the elevation in boiling point. The liquid solid boundary is lower for the solution indicating the depression in freezing point. The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm. Phase diagram of pure solvent and solution. - ResearchGate Phase diagram of pure solvent and solution. Source publication Design and Evaluation of Multiple Effect Evaporator Systems According to the Steam Ejector Position Article Full-text available Jan...
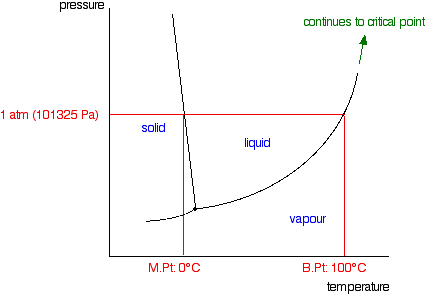
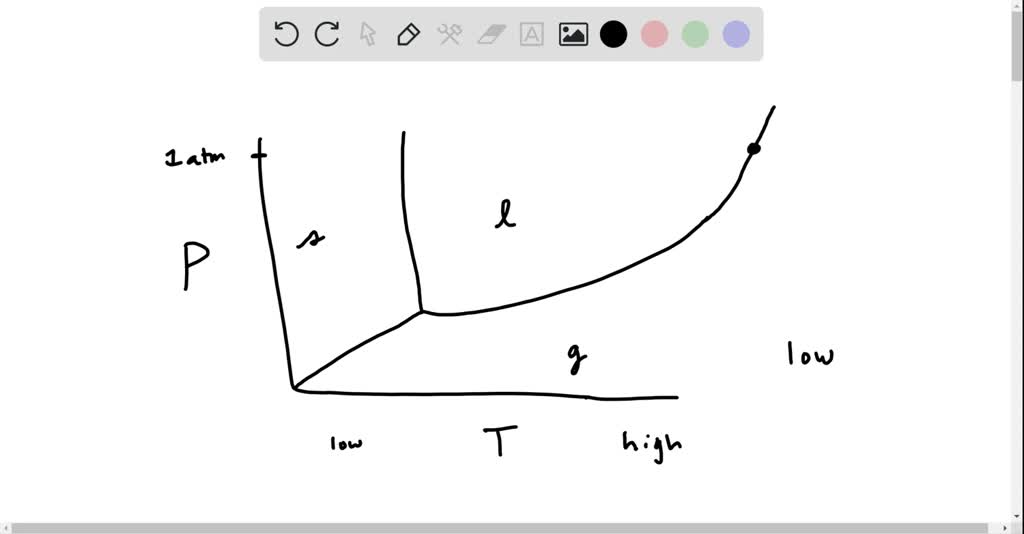
phase diagrams of pure substances - chemguide A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
1.0: Phase Diagrams of Pure Substances - Chemistry LibreTexts A phase diagram is a graphic summary of the physical state of a substance as a function of temperature and pressure in a closed system. Introduction A typical phase diagram consists of discrete regions that represent the different phases exhibited by a substance (Figure 1.0. 1 ).
Ternary phase diagrams of ethyl paraben and propyl paraben in ethanol ... A pure water solution saturated by certain paraben is homogenous from 1.0 °C to 40.0 °C, but at 49.4 °C the system of butyl paraben in water turns into a system of two liquid phases (an aqueous phase with a low concentration of butyl paraben and a second liquid phase with a high butyl paraben concentration).
The phase diagrams for the pure solvent (solid lines) and the solution ... asked Dec 24, 2021 in Chemistry by SupriyaRani (24.2k points) The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by L in the figure is: A. Δp Δ p B. ΔT f Δ T f C. K bm K b m D. K f m K f m class-12 graphical-interpretation
Phase and Phase System (Solution Solvent Solute) Differentiate between solvent and solute. Ans. Solution: A one phase homogeneous mixture of two or more kinds of different molecular or ionic substance is called a solution. Solvent. The component of the solution which is present in large quantity is called solvent. Solute . The component in small quantity is called a solute. Example: In a ...
Phase diagram of Carbon - SlideServe P1 = X1 P1Raoult's Law o o If P1 is the vapor pressure of the solution, P1 the vapor pressure of the pure solvent, and X1 the mole fraction of the solvent in the solution,then: Most solutions obey Raoult's law at low concentrations of solute. Deviations from Raoult's law provides information on the nature of the solute-solvent interactions.
(PDF) [Easterling, Kenneth E.; Porter, Phase ... - Academia.edu [Easterling, Kenneth E.; Porter, Phase Transformations in Metals and Alloys. Juan jimenez flores. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 37 Full PDFs related to this paper. Download. PDF Pack. People also downloaded these PDFs. People also downloaded these free PDFs . People also downloaded …
8.2: Phase Diagrams of Pure Substances - Chemistry LibreTexts The diagram shows seven different solid phases of H 2 O differing in crystal structure and designated ice I, ice II, and so on. Ice I is the ordinary form of ice, stable below 2bar. On the diagram are four triple points for two solids and the liquid and three triple points for three solids. Each triple point is invariant.
Figure 1 below shows the phase diagram of a solution - Course Hero Figure 1 below shows the phase diagram of a solution and the effect that the lowered vapor pressure has on the boiling point of the solution compared to the solvent. In this case the sucrose solution has a higher boiling point than the pure solvent. Since the vapor of the solution is lower, more heat must be supplied to the solution to bring its vapor pressure up to the pressure of the ...
SOLVED: The phase diagrams for = pure solvent and the solvent solution ... Identify the normal freezing ( (pwolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpsoln) points of the solution at atm_ Assume the solute nonvolatile and that the solid that freezes from solution pure solvent. atm Liquid 1 L Solid Answcr Bonk bpan Inaeln Gas Temperature pal Discussion
The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i...
Phase Transition: Definition, Examples, Types, Important Questions 13.04.2022 · Every pure element in the periodic table has a normal melting point, which is the temperature at which the element converts into liquid under 1 atmosphere of pressure. Boiling Every substance also has a boiling point. The boiling point is the temperature at which a liquid turns into a gas. The boiling point changes with temperature and pressure. Like melting point, …
Binary Solid-Liquid Phase Diagram | Chem Lab From your data, generate the solid-liquid phase diagram for your system and determine Δ fusH and the melting point for the two pure substances at 95% confidence. Determine the eutectic temperature and composition, along with your best estimate of the uncertainties for these values.
Polarities of Solvents | Shodex/ HPLC Columns, Detectors, … Solvent-peak Separation Columns To Realize Pure SEC Mode Guidelines for Shodex Column Selection Sample Concentration for SEC Analysis Effects of Sample Load (Pullulan) Selection of Calibration Curves Selection of Calibration Standards Connection of Different Pore-size Columns Comparison of Calibration Curves: Linear Type and Conventional Type Comparison of …
Solved Label the phase diagram of pure solvent and a | Chegg.com Expert Answer. 100% (138 ratings) This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
CH150: Chapter 7 – Solutions – Chemistry - Western Oregon … 7.1 Introduction: Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Solutions are all around us. Air, for example, is a solution. If you live near a lake, a river, or an ocean, that body of water is not pure H 2 O but most probably a solution.
The phase diagrams for the pure solvent (solid lines) and the solution ... Get an expert solution to The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by 'L' in the figure is. ... Get Instant Solutions. When in doubt download our app. Now available Google Play Store- Doubts App . Download Now. Recieve an sms with ...
PDF Phase Diagrams, Solid Solutions, Phase Transformations Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
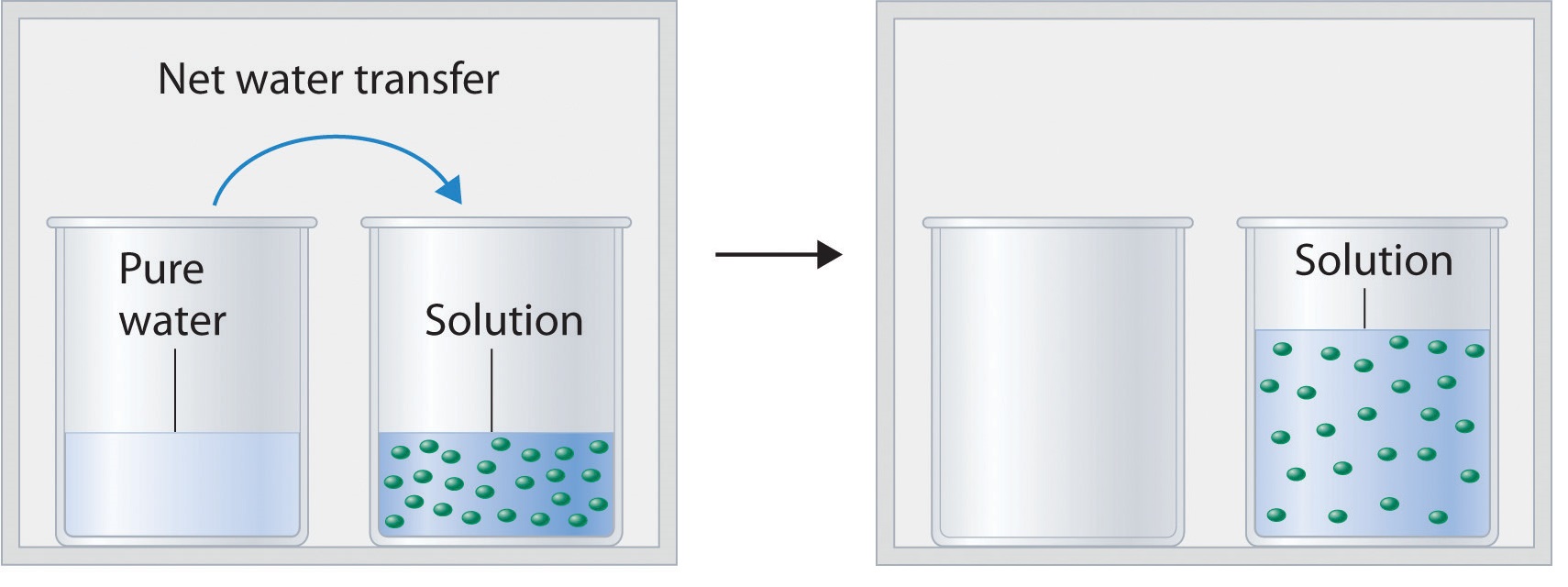
Solution (chemistry) - Wikipedia In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent.The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation.
PDF Phase Diagrams The phase diagram in Figure 1 is for a pure compound. When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature ...
Phase (matter) - Wikipedia In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform.: 86 : 3 Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, …
PDF phase diagram solvent solution - Just Only phase diagram solvent solution Effect of Solute on Phase Diagram of Water SOLIDLIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTfΔTb ΔP Temperature
What are the most important differences between the phase di - Quizlet Two differences we can see on phase diagrams of a pure solvent and a solution are changes in phase transition temperatures \\textbf{phase transition temperatures} phase transition temperatures.Solution will always have a higher boiling point \\textbf{higher boiling point} higher boiling point and lower freezing point \\textbf{lower freezing point} lower freezing point (check pages 501, 502).




















Post a Comment for "39 phase diagram of pure solvent and solution"