40 examples of exempt human specimens
Preparation and Collection of Specimens | Monkeypox | Poxvirus | CDC Specimens should be collected in the manner outlined below. When possible, use a plastic, sterile, leak-proof container rather than glass materials for specimen collection. Two swabs from each lesion (in general, 2-3 lesions should be sufficient) should be collected for testing. Using two sterile synthetic swabs (including, but not limited to ... Research Associate in Worcester, Massachusetts | Careers at SCHOOL POSITION SUMMARY: We are seeking a Research Associate to work collaboratively on CRISPR-Cas gene editing of the Huntington's Disease (HD) gene. HD is a devasting neurodegenerative disease that currently has no effective treatment. Our goal is to develop gene editing methods to inactivate or correct the disease-causing version of the gene at the DNA level. We are looking for ambitious ...
The Importance of Museum History - Whippanyrailwaymuseum.org In the 340s BCE, he traveled to the island of Lesbos and studied the specimens there. Aristotle later founded his philosophical school in Athens, which included a library and mouseion. Art in museums. Art in museums is a powerful way to learn about human culture and history.
Examples of exempt human specimens
Clinical Laboratory Scientist Generalist at Sutter Health EDUCATION. Bachelor's: degree in biology or related science. CERTIFICATION & LICENSURE. CA Licensed Clinical Laboratory Scientist. EXPERIENCE. 2 years experience as a CLS Generalist Trainee or a Limited CLS Trainee. SKILLS AND KNOWLEDGE. Demonstrated knowledge and technical competence in routine specimen collection, preparation, testing ... Clinical Lab Scientist II - Molecular (Short Hour) at Sutter Health Non-Exempt. Weekly Hours: 16. Employee Status: Short Hour. Number of Openings: 1. This position may regularly work, store, prepare, receive, unpack, transport, dispose of, or administer drug(s) identified as hazardous, or potentially hazardous, by the National Institute for Occupational Safety and Health (NIOSH) for purposes of USP 800. IT Job Technology Managers provide structure, guidance and support for budgeting, capital, and Opex planning for delivery of technology. May serve as Secretary, Assistant Secretary or member of IT for institutional committees. The ideal candidate will have a passion for Front-End Engineering, both the technology and the community surrounding it.
Examples of exempt human specimens. Current Best Practices for Generalizing Sensitive Species ... - GBIF The unprotected distribution of Sensitive Primary Species Occurrence Data (for example the exact localities of rare, endangered or commercially valuable taxa) was a concern of GBIF - the Global Biodiversity Information Facility - from its beginning. The GBIF Secretariat has a vested interest in making data available via its portals, but at the same time respecting the wishes of data ... Johns Hopkins University Research Technologist in Baltimore, MD ... - Snag Research Technologist The Pathology Department is seeking a Research Technologist which is a project based position in support of 2021-2022 initiative for COVID 19 testing at Johns Hopkins.This role will handle COVID-19 and related human specimens following training, standard operating, and safety protocols. List of culture media used in microbiology with their uses Most commonly used media for culturing urine samples. · Egg Saline Medium. Preservation of cultures of Gram-negative bacilli. · Egg Yolk Agar. Detection of lipase and lecithinase activity of Clostridium species. · Ellner's Medium. · Medium of Duncan and Strong. · Medium of Phillips. · Alkaline Egg Medium. WHMIS 2015 - Laboratories : OSH Answers - Canadian Centre for ... Suppliers and employers must use and follow the new WHMIS 2015 requirements for labels and safety data sheets (SDSs) for hazardous products sold, distributed, or imported into Canada. Please refer to the following OSH Answers documents for information about WHMIS 2015: WHMIS 2015 - General. WHMIS 2015 - Pictograms. WHMIS 2015 - Labels.
› ohrp › regulations-and-policyEngagement of Institutions in Human Subjects Research (2008) Once an activity is determined to involve non-exempt human subjects research, this guidance should be used to determine whether an institution involved in some aspect of the research is engaged in that human subjects research, because if it is, certain regulatory requirements apply. Specifically, institutions that are engaged in non-exempt ... Instructional Guidance and Sources for Human Subject Investigators ... The following documents and information sources are to assist investigators as they are designing or modifying their protocols (i.e. guidance materials). To access fillable templates and forms, please visit the "IRB Forms" page (or click here to redirect you to the Forms page). NTR IRB has created two 15-minute training videos to assist you in Read More » › ohrp › regulations-and-policyCoded Private Information or Specimens Use in Research ... Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ... grants.nih.gov › policy › humansubjectsHuman Subjects Research - Home page | grants.nih.gov Aug 02, 2021 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections.
What does tyrannical government mean? - askingforanswer.com An example of tyranny is someone putting someone in jail for years for a small crime. noun. The definition of tyranny is a government or ruler with total power. ... The rule of law is the principle that no one is exempt from the law, even those who are in a position of power. ... or the cloning of such specimens, with the intent to be used as ... General Eligibility Requirements for Hospital apply for Joint ... Hospitals that primarily conduct non-human subjects research and/or research exempt from review by an IRB or research ethics committee, such as medical record review studies, case studies, and research involving data/specimens without individually identifiable information, do not meet criterion 3 of the academic medical center hospital ... Article - Office of Human Research Et... Except as provided in Section 2.10 of these procedures, informed consent must be documented by the use of a written consent form approved by the IRB. For exempt research, requirements regarding the consent process are outlined in the Office of Human Research Ethics (OHRE) SOP 601: Exempt Studies. Improving Clinical Trial Sampling for Future Research - Medscape In July 2011, the US Department of Health and Human Services issued an Advanced Notice of a Proposed Rulemaking (ANPRM) aimed at enhancing this regulation, with many proposals that would directly ...
grants.nih.gov › grants › how-to-apply-applicationG.500 - PHS Human Subjects and Clinical Trials Information Oct 25, 2021 · Note for studies involving only the secondary use of identifiable biospecimens or data: For studies where the only involvement of human subjects is the use of identifiable biospecimens or data originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials Information form with information specific to the current study and not the original collection unless the ...
Overview of Testing for SARS-CoV-2, the virus that causes COVID-19 The U.S. Department of Health and Human Services has required laboratories and testing facilities to report race and ethnicity data to health departments, in addition to other data elements, for individuals tested for SARS-CoV-2 or diagnosed with COVID-19. Healthcare providers and public health professionals need to ask and record race and ...
CLIA 101: Answers to your most common questions about CLIA waived tests The CLIA program regulates labs that perform laboratory testing (and diagnostics) on human specimens to maintain the accuracy, reliability and reporting of patient tests and results. It regulates specific types of tests performed, as well as the training and education of personnel, quality control and the timeliness and accuracy of test results.
› Standards-Ethics › InstitutionalIRB FAQs for Survey Researchers - AAPOR According to the Federal regulations (45 CFR 46.101(b)), survey research may be exempt from the regulations unless "the information obtained is recorded in such a manner that the human subjects can be identified, directly or through identifiers linked to the subjects" or if "federal statute(s) require(s) without exception that the ...
Position Description Supervisory Certification: I certify that this is an accurate statement of the major duties and responsibilities of this position and its organizational relationships, and that the position is necessary to carry out Government functions for which I am responsible. This certification is made with the knowledge that this information is to be used for statutory purposes relating to appointment ...
IT Job Job Description. Mayo Clinic is the nation's best hospital (U.S. News & World Report, 2021-2022) and ranked #1 in more specialties than any other care provider. We have a vast array of opportunities ranging from Nursing, Clinical, to Finance, IT, Administrative, Research and Support Services to name a few.
RFA-NS-23-017: Optimization of Genome Editing Therapeutics for ... For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials.
NIH Policy Manual a. Non-exempt human subjects research conducted in a foreign country with a foreign institution, must be with an institution that holds an active FWA and reviewed by an IRB/Ethics Committee (EC) registered with the HHS Office for Human Research Protections (OHRP) or has equivalent procedures consistent with 45 CFR 46.101(h).
Corrections Agent (Probation Officer), Community Corrections Division ... ‹ Back to Listings Page Corrections Agent (Probation Officer), Community Corrections Division, Human Services Department. Stearns County • Unavailable, MN Monster • August 17, 2022 • August 17, 2022
Transport of Human Remains and Biological Specimens Human Ashes. Ø Death certificate or true copy thereof reviewed and signed by the City Health Officer. Ø Cremation Certificate stating that the human remains have been properly cremated and ashes shall be placed in a hermetically sealed urn or a similar container. Ø Transfer permit from the City Health Office. c.) Human Bones.
Types of IRB Review Free Essay Example - studybounty.com Essay Sample The three types of IRB review are exempt, expedited, and full board. Exempt review is the quickest and easiest, while full board review is the most time-consuming. ... records and specimens if they are available (Whitney et al. 2008). ... J. F., & Lo, B. (2005). Human subjects issues and IRB review in practice-based research. The ...
PART 866 - IMMUNOLOGY AND MICROBIOLOGY DEVICES Authority: 21 U.S.C. 351, 360, 360c, 360e, 360j, 360l, 371. Source: 47 FR 50823, Nov. 9, 1982, unless otherwise noted ...
⚾|【MLB】プホルスに2本差に迫られたAロッドが現役復帰? "宣戦布告"に反響「700本打って」 - PORTALFIELD News aロッドはsnsに動画を投稿、現役復帰をほのめかして話題に今季限りでの現役引退を表明しているカージナ…
Biological Hazard Examples and Safety Levels | SafetyCulture 1. Human blood and blood products. Bodily fluids, tissues that contain blood, serum, plasma, and other blood components in liquid or semi-liquid form are examples of biological hazards. 2. Animal waste. Any animal body part or the beddings of infected animals are also considered as biological hazards. 3.
Exempt Review - North Texas Regional Institutional Review Board ALL research projects involving human subjects require a review by the North Texas Regional Institutional Review Board (IRB). Note that this review process may involve several stages or levels of review, depending on the various features of the research project and the potential risk to the subjects. What is Exempt Category Review of Research Studies Read More »
researchintegrity.asu.edu › human-subjectsHuman subjects | Research Integrity and Assurance All institutions engaged in human subjects research that is not exempt from 45CFR46, and is conducted or supported by any HHS agency must be covered by an Office for Human Research Protections(OHRP)-approved assurance of compliance. The Federalwide Assurance (FWA) is the only type of assurance accepted and approved by OHRP.
irb.northwestern.edu › exempt-reviewExempt Review: Institutional Review Board (IRB) Office ... Exempt Review; Exempt Review. Exempt human subjects research is a specific sub-set of “research involving human subjects” that does not require ongoing IRB oversight. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal ...
IT Job Technology Managers provide structure, guidance and support for budgeting, capital, and Opex planning for delivery of technology. May serve as Secretary, Assistant Secretary or member of IT for institutional committees. The ideal candidate will have a passion for Front-End Engineering, both the technology and the community surrounding it.
Clinical Lab Scientist II - Molecular (Short Hour) at Sutter Health Non-Exempt. Weekly Hours: 16. Employee Status: Short Hour. Number of Openings: 1. This position may regularly work, store, prepare, receive, unpack, transport, dispose of, or administer drug(s) identified as hazardous, or potentially hazardous, by the National Institute for Occupational Safety and Health (NIOSH) for purposes of USP 800.
Clinical Laboratory Scientist Generalist at Sutter Health EDUCATION. Bachelor's: degree in biology or related science. CERTIFICATION & LICENSURE. CA Licensed Clinical Laboratory Scientist. EXPERIENCE. 2 years experience as a CLS Generalist Trainee or a Limited CLS Trainee. SKILLS AND KNOWLEDGE. Demonstrated knowledge and technical competence in routine specimen collection, preparation, testing ...






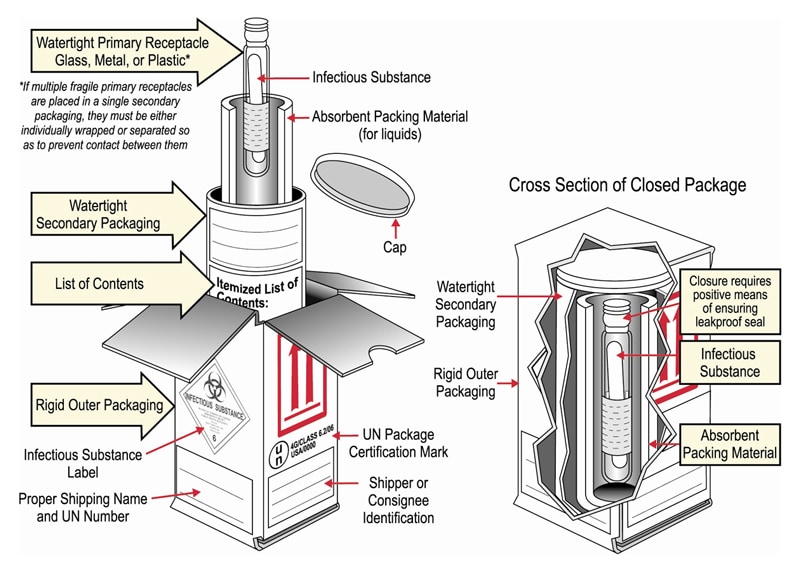







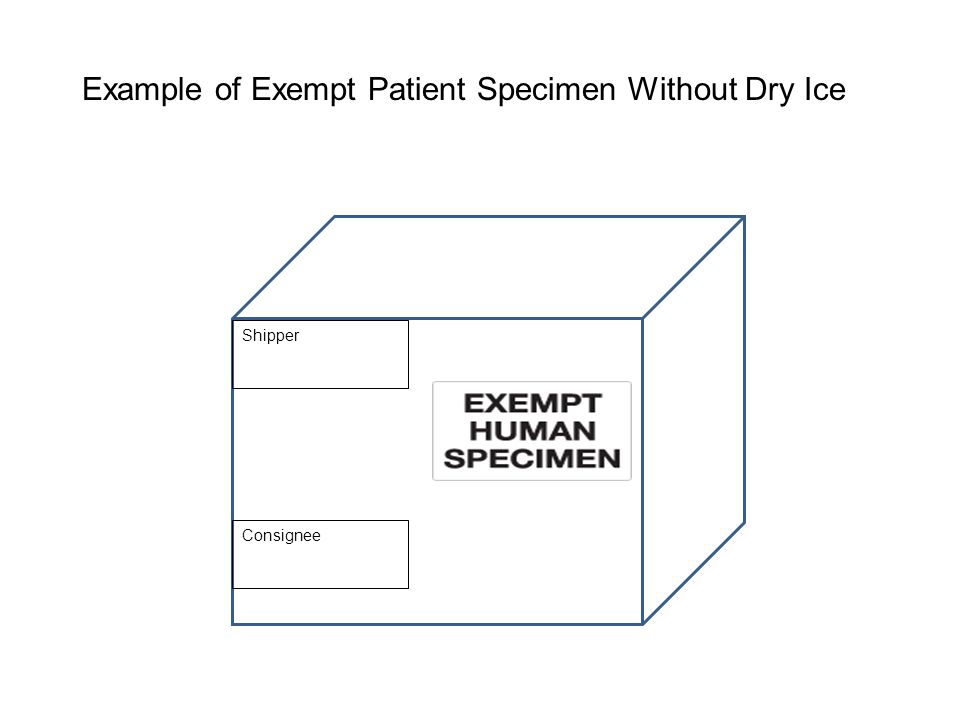


Post a Comment for "40 examples of exempt human specimens"