40 which health claim on a food label is not allowed
Nutrition claims - Language selection | Food Safety A claim that a food does not contain saturated fat, and any claim likely to have the same meaning for the consumer, may only be made where the sum of saturated ... New Rules on Food Health Claims | Food Processing Foods that carry the whole-grain health claim are required to contain 51 percent or more whole-grain ingredients by weight, and they must also be low in fat. There is no current test to measure the whole-grain levels the FDA approves for whole-grain claims (fiber content is used instead).
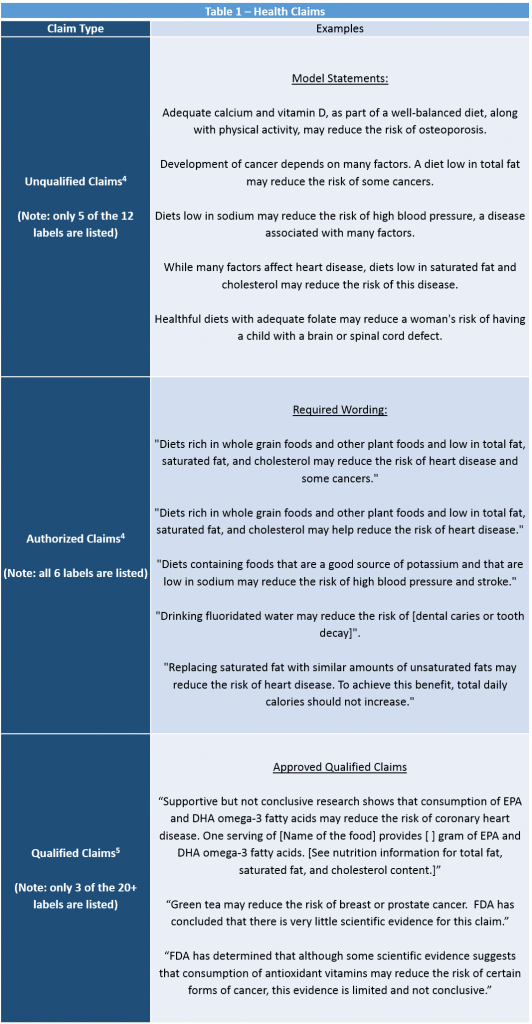
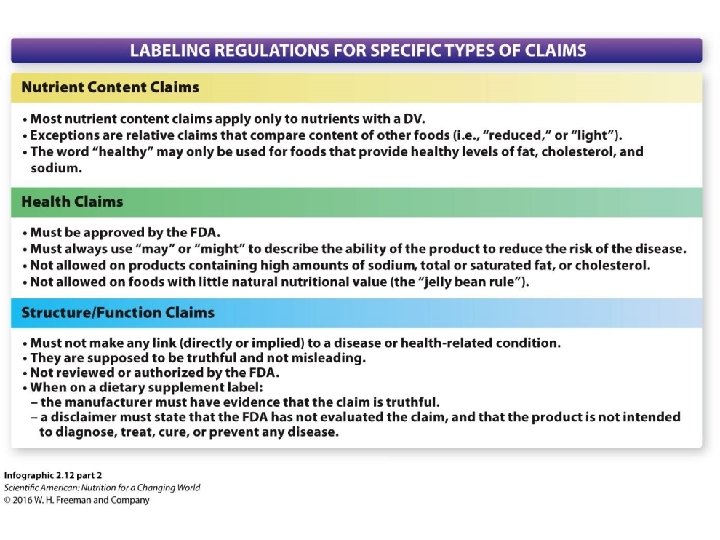
General Food Labeling Requirements - California VerkkoHealth Claims . A “ Health Claim ”is a food label message that describes the relationship between a food component, such as fat, calcium, or fiber, and a disease or health-related condition. FDA has approved various health claims based on extensive scientific evidence and defined conditions under which the claims can be used

Which health claim on a food label is not allowed
Nutrition and health claims: guidance to compliance with Regulation (EC ... New GB nutrition claims. The appropriate UK authorities may, after consulting an expert committee, amend the list of permitted nutrition claims contained within the Annex to Regulation (EC) No ... Method of production claims on food labels - Canadian Food Inspection ... For example: a food may be fortified with vitamins or contain an ingredient or additive that has been sourced from a plant or animal and/or derived from minimum processes.While the guidance provided for natural claims would not normally recognize a food to be natural if it contains added vitamins or additives (for example, milk with added vitamins A and D, enriched flour), companies would ... Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Which health claim on a food label is not allowed. Dietary Supplement Label Claims: What's Allowed? What's Not Allowed? It is illegal for a company selling a dietary supplement to promote or label their product to diagnose, cure, mitigate, treat or prevent any disease. A Health Claim is an explicit or implied characterization of a relationship between a substance and a disease or health related conditions and must be approved by the FDA. Nutrition content claims and health claims - Food Standards All health claims must be supported by scientific evidence. Health claims are only permitted on foods that meet the Nutrient Profiling Scoring Criterion (NPSC). For example, the Standard doesn't allow health claims on foods higher in saturated fat, sugar or salt. There are 2 types of health claims - general and high level. Dietary Supplements - Claims and Labeling Compliance for FDA/FTC We can review or prepare labels and advise your company regarding products requiring FDA/FTC-compliant labeling such as dietary supplements, nutraceuticals, or cosmetics. We will assist with FDA compliance regarding foods, dietary supplements, drugs, or medical devices. We can determine whether product names and claims cause the product to meet ... Health Claims on Food Labels - LabelCalc A lot of new food manufacturers ask me about making health claims on food labels, so today I wanted to shed some light on what can sometimes be a complex process. In order to successfully navigate the world of claims for your food product—from health claims to nutrient content claims and everything in between—here are the FDA rules you need ...
Which health claim on a food label is not allowed? - Quora Such claims are supported by scientific evidence and may be used on conventional foods and on dietary supplements to characterize a relationship between a substance (a specific food component or a specific food) and a disease or health-related condition (e.g., high blood pressure). Food Packaging Claims | American Heart Association You can use this general guidance: "Free" means a food has the least possible amount of the specified nutrient. "Very Low" and "Low" means the food has a little more than foods labeled "Free." "Reduced" or "Less" mean the food has 25% less of a specific nutrient than the regular product. Decoding the Nutrition Label: Health Claims and Nutrient Content Claims Many Canadians use the nutrition label to find out about the amount of calories or nutrients found in a certain food. The nutrition label has plenty of information to help you make healthy choices at the grocery store. The nutrition label includes the Nutrition Facts table, the ingredient list, health claims and nutrient content claims. Health claims and nutrient content claims are two tools ... Glyphosate | US EPA Verkko8.9.2022 · Human Health. EPA scientists performed an independent evaluation of available data for glyphosate and found: No risks of concern to human health from current uses of glyphosate. Glyphosate products used according to label directions do not result in risks to children or adults. No indication that children are more sensitive to …
Misleading Nutrition and Food Labels - Health You're not alone. Nearly 59% of consumers have a hard time understanding nutrition labels, according to a Nielsen survey. Here's our list of the 16 most common—and most misleading phrases ... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Food labelling and packaging: Nutrition, health claims and supplement ... You cannot claim or imply that food can treat, prevent or cure any disease or medical condition. Food supplements, fortified foods and foods for specific nutritional uses You must follow certain... Health Claims - Canada.ca Health claims are also subject to Section 3 of the Food and Drugs Act that prohibits the labelling and advertising of any food to the general public, as a treatment, preventative or cure for any diseases and health conditions listed in Schedule A of the Food and Drugs Act.
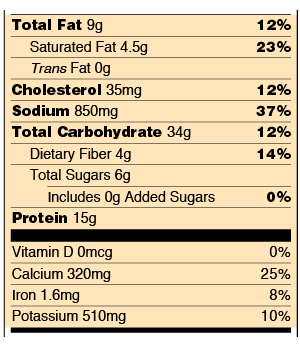
FDA Regulatory Requirements for Nutrient Content Claims When a claim is made on a food that contains more than 13 g total fat, 4 g saturated fat, 60 mg cholesterol, or 480 mg sodium per RACC, per labeled serving, or, for foods with small RACC, per 50 g, a disclosure statement is required as part of claim (i.e., “See nutrition information for ___ content” with the blank filled in with nutrient(s) that exceed the prescribed levels).
How to Read a Dog Food Label – American Kennel Club Verkko30.11.2020 · The Flavor Rule: According to the U.S. Food and Drug Administration (FDA), if the label says “Beef Flavor Dog Food,” then “a specific percentage (of the beef) is not required, but a product ...
Nutrition Flashcards | Quizlet Associated in a reliable health claim allowed on food labels. Tomatoes and prostate cancer. Associated in a health claim NOT allowed on food labels without a disclaimer. Epiglottis. prevents food from entering the windpipe when swallowing. Pyloric Sphincter. Controls the entry of chyme into the duodenum.
Which claim on a food label is not allowed a a - Course Hero 72) Which claim on a food label is NOT allowed? a) A healthy diet rich in fruits and vegetables may reduce the risk of some types of cancer.
Health claims - Food Safety A health claim is any statement about a relationship between food and health. The Commission authorises different health claims provided they are based on scientific evidence and can be easily understood by consumers. The European Food Safety Authority (EFSA) is responsible for evaluating the scientific evidence supporting health claims.
Health and nutrition claims - EU labelling rules 18 May 2022 — be false, ambiguous or misleading · cast doubt on the safety or nutritional adequacy of other foods · encourage or condone excess consumption of a ...
Nutrition, health and related claims - Food Standards Health claims You can only base health claims on food-health relationships that have been substantiated according to Standard 1.2.7. All health claims must be supported by scientific evidence to the same degree of certainty, whether they are pre-approved by us or self-substantiated by food businesses. General level health claims
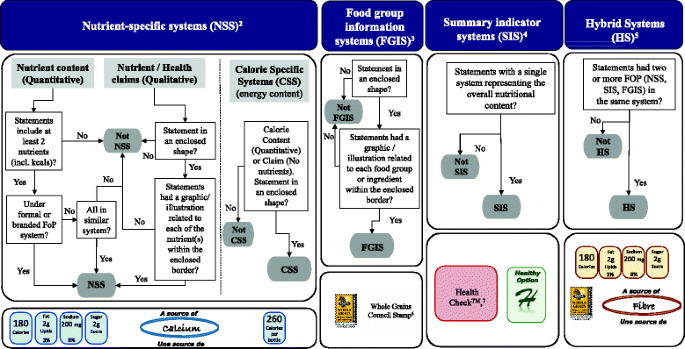
GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS Verkko2.1.2 Nutrient comparative claim is a claim that compares the nutrient levels and/or energy value of two or more foods. (Examples: “reduced”; “less than”; “fewer”; “increased”; “more than”.) 2.1.3 Non-addition claim means any claim that an ingredient has not been added to a food, either directly or indirectly.
Health claims on food labels - Food labels - Canadian Food Inspection ... Section 3 of the Food and Drugs Act (FDA), prohibits the sale of a food that is labelled or advertised to the general public as a treatment, preventative or cure for any of the diseases referred to in Schedule A, unless exempted within the Food and Drug Regulations (FDR).
In Pictures: 29 Foods With “Health Claims” That Are Deceiving … Verkko26.4.2013 · Seeing as apparently 47% of Americans agree with this statement that “everything causes cancer” (in other words, there’s ambiguous information on what’s healthy food) I did some research. Naturally, I took my research to the grocery store. Foods With Health Claims: The Surprising Ways That Food Companies Try to Trick You
Food composition and quality claims - Canadian Food Inspection … VerkkoThe claim "not roasted in oil", on the other hand, is an implied claim regarding the fat content of the food and may only be made if implied nutrient content claim principles are satisfied. For example, the claim "not roasted in oil" may be acceptable if preceded or followed by a reduced in fat claim on a product that meets the conditions of this claim.
Nutrition and health claims on labels and in food advertising 28 Feb 2022 — Examples: "contains no trans fatty acids" or "cholesterol free" are not permitted as they are not listed in the Annex; "low in carbohydrates" or ...
Nutrient Claims on Food Labels - HGIC@clemson.edu Implied: These claims are prohibited if they wrongfully suggest that there is, or is not, a meaningful level of a nutrient in a food. For example, "made with oat bran" is not allowed unless the product contains enough oat bran to meet the definition of "good source" of fiber.
Using Dietary Supplements Wisely - NCCIH VerkkoHealth claims describe a relationship between a substance in the supplement and reduced risk of a disease or condition. They must be based on scientific evidence. For example, if a supplement label says “Calcium may reduce the risk of the bone disease osteoporosis,” that’s a health claim.
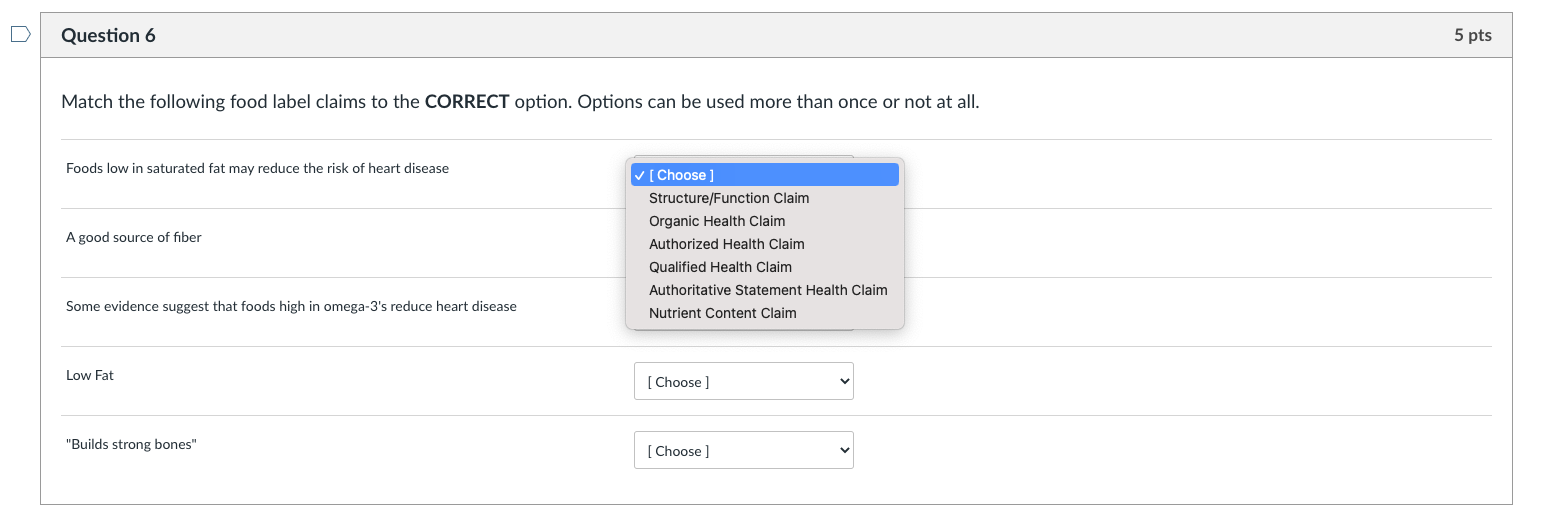
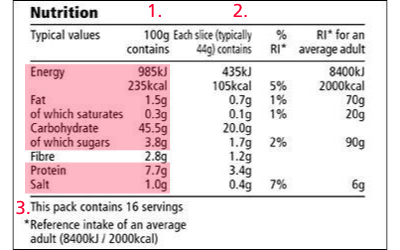
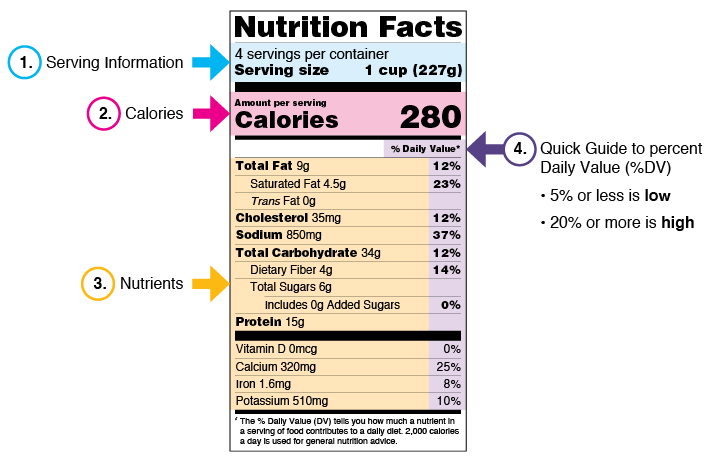
5 Understanding Food Labels and Health Claims - Maricopa low salt. Fewer than 140 milligrams of sodium. low cholesterol. Fewer than 20 milligrams cholesterol and 2 grams of saturated fat. lean. FEver than a set amount of grams of fat for that particular cut of meat. high. It contains more than 20% of the nutrient's daily value. good source.
Label Claims for Conventional Foods and Dietary Supplements Verkko7.3.2022 · There are three ways in which FDA exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990 ...
What health claim on a food label is not allowed? - Vigor Tip What health claim on a food label is not allowed? Health claims for the treatment, prevention or cure of diseases such as Alzheimer's disease and cancer are not permitted on food products. These are considered drug claims. What are the four types of health claims found on food labels? There are 4 main types of product claims…
Questions and Answers on Health Claims in Food Labeling VerkkoHealth claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a health-related ...
EUR-Lex - 32006R1924 - EN - EUR-Lex Verkko(4) This Regulation should apply to all nutrition and health claims made in commercial communications, including, inter alia, generic advertising of food and promotional campaigns, such as those supported in whole or in part by public authorities.It should not apply to claims which are made in non-commercial communications, such as dietary …
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
Chapter 2 T.B. Flashcards - Quizlet Which claim on a food label is not allowed? a) Diets low in fat and rich in fruits and vegetables may reduce the risk of some types of cancer.
Chapter 2 T.B. - Flashcards | StudyHippo.com Which claim on a food label is not allowed? a) Diets low in fat and rich in fruits and vegetables may reduce the risk of some types of cancer. b) Diets rich in vitamin C will reduce the incidence of colds and flu c) Adequate calcium intake throughout life helps maintain bone health and reduce the risk of osteoporosis.
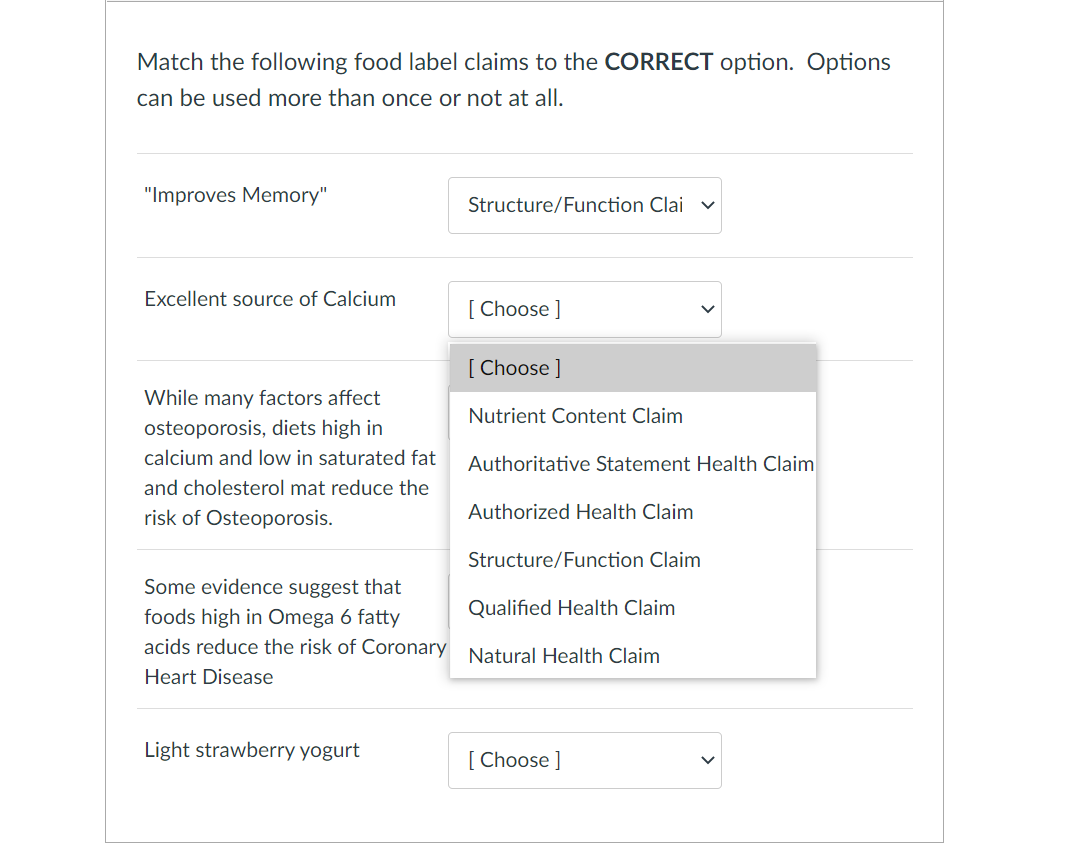
health claims and food labels Flashcards | Quizlet -cholesterol must be declared if it is 2mg or more nutrient content claims It is a claim on a food product that directly or by implication characterizes the level of a nutrient in the food etc: low (lo), high (hi), free, without, no, zero structure function claims -role of a nutrient in humans -can't mention a disease -"calcium builds strong bones"
Which health claim on a food label is not allowed? - Quora Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce ...
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Method of production claims on food labels - Canadian Food Inspection ... For example: a food may be fortified with vitamins or contain an ingredient or additive that has been sourced from a plant or animal and/or derived from minimum processes.While the guidance provided for natural claims would not normally recognize a food to be natural if it contains added vitamins or additives (for example, milk with added vitamins A and D, enriched flour), companies would ...
Nutrition and health claims: guidance to compliance with Regulation (EC ... New GB nutrition claims. The appropriate UK authorities may, after consulting an expert committee, amend the list of permitted nutrition claims contained within the Annex to Regulation (EC) No ...


:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)


















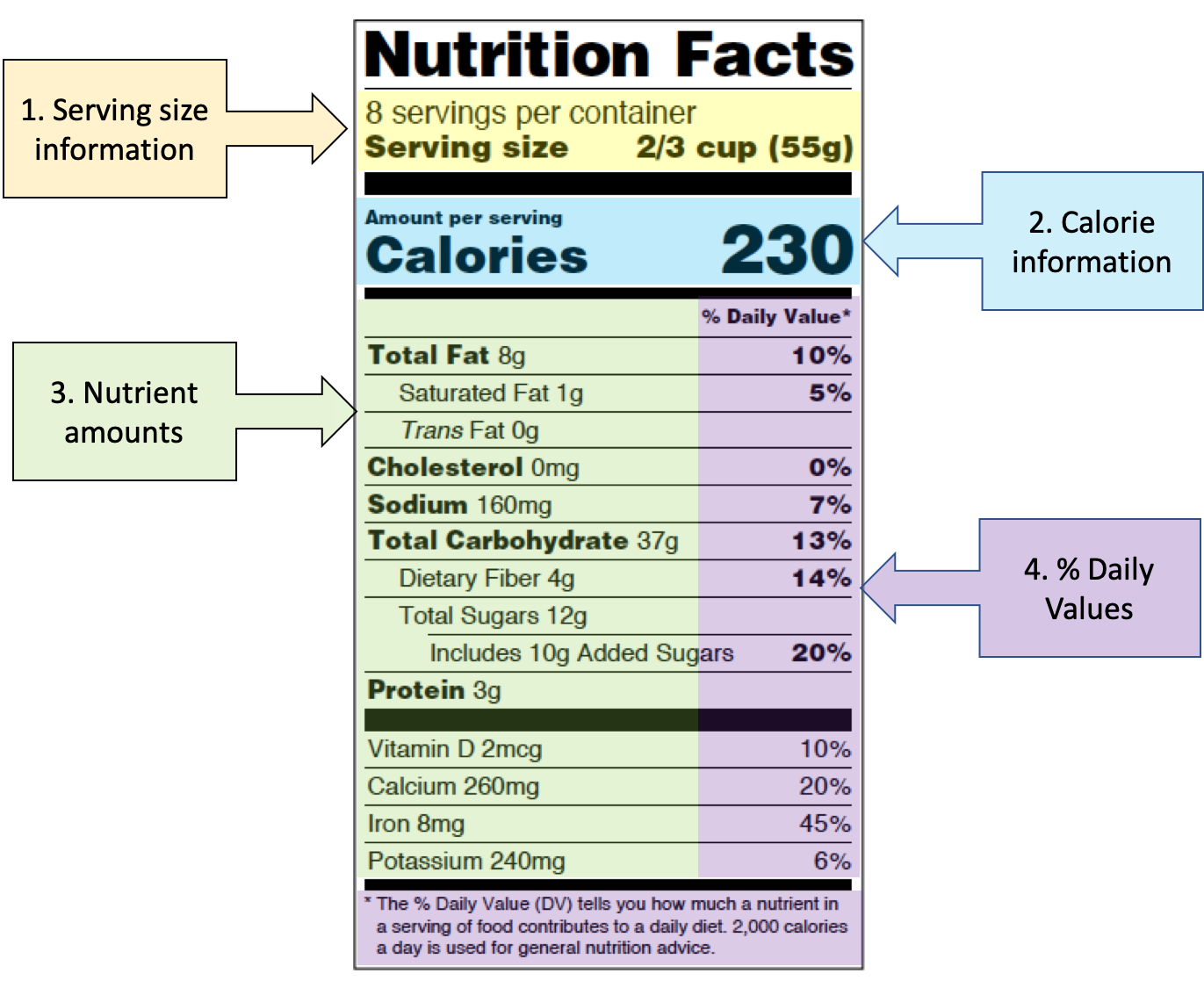

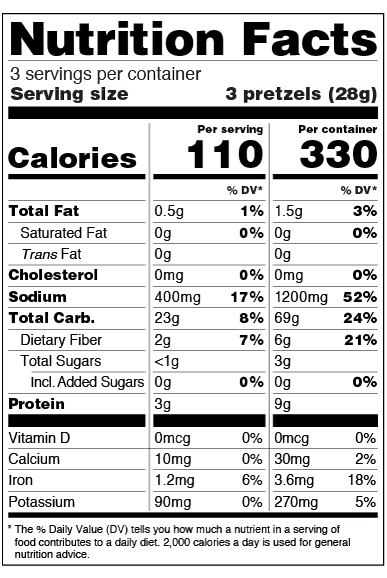



Post a Comment for "40 which health claim on a food label is not allowed"