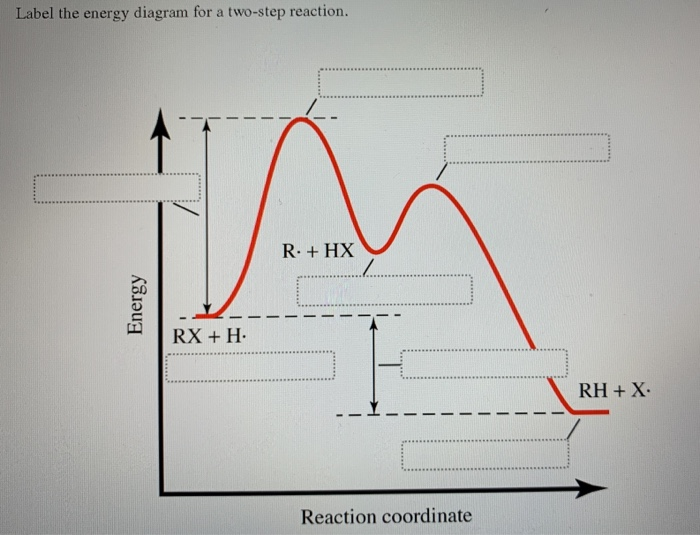
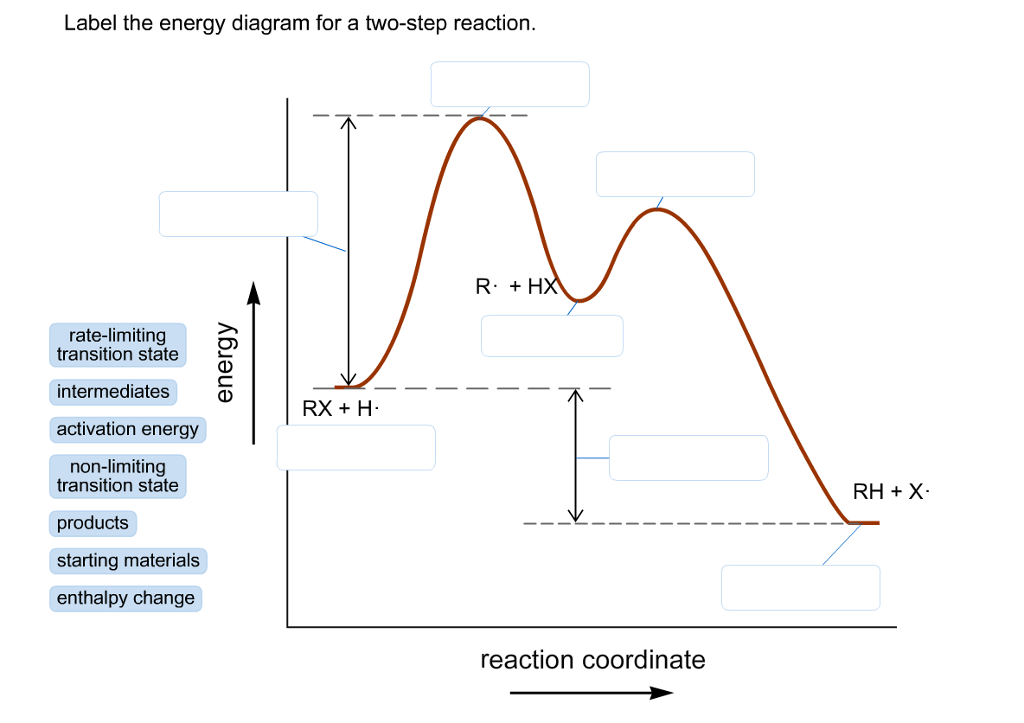
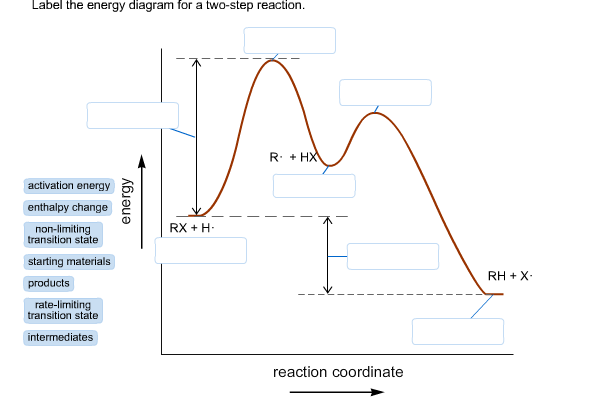
42 label the energy diagram for a two‑step reaction.
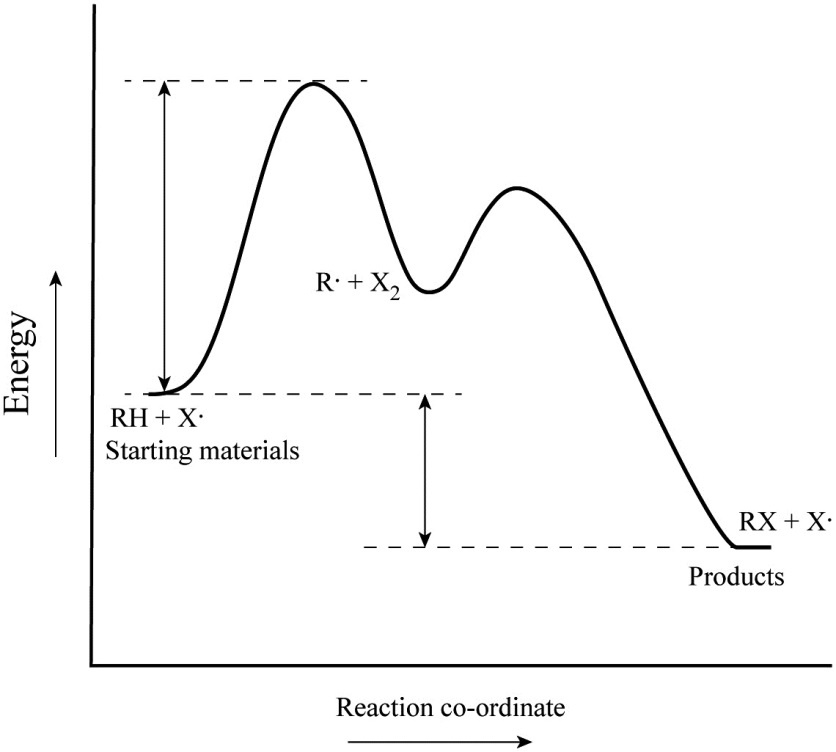
Energy Diagram for a Two-Step Reaction Mechanism A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it ... label the energy diagram for a two-step reaction. - OneClass Draw an energy diagram for a two-step reaction where the first step is exothermic and the reaction overall is endothermic. The first step is rate limiting. Label reactants, products and intermediates
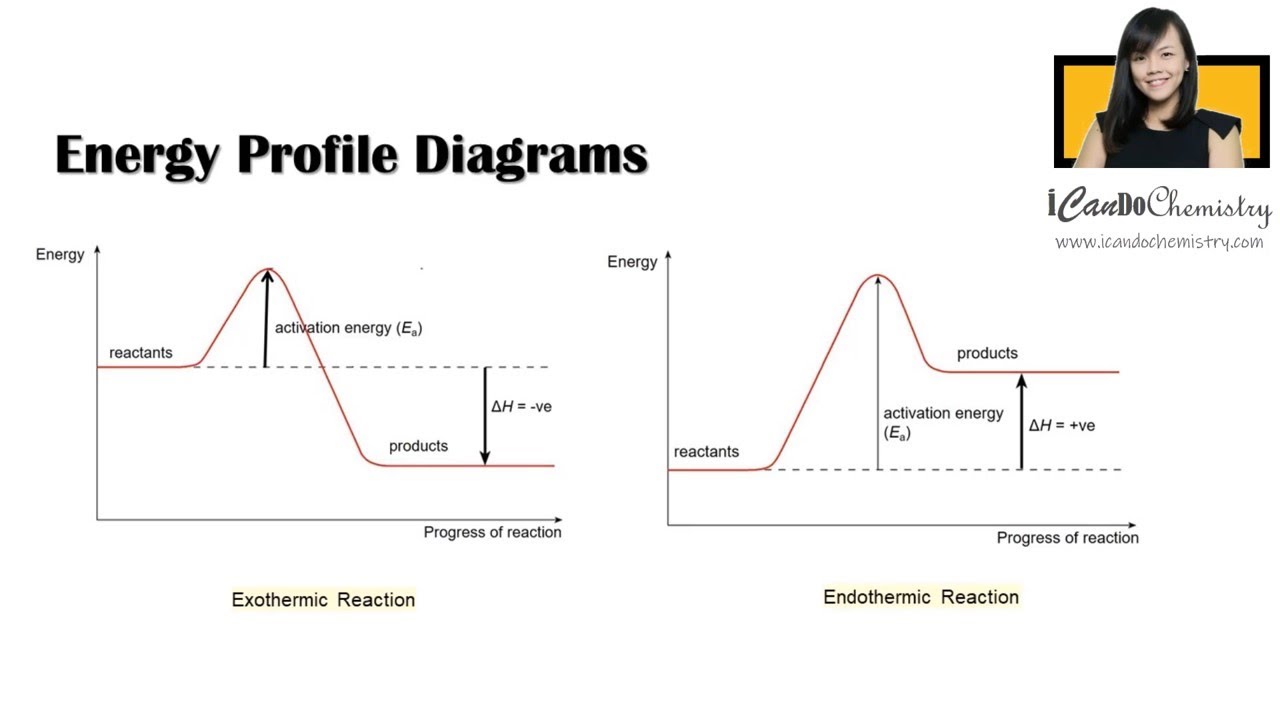
Energy Diagram — Overview & Parts - Expii Helpful Steps to Create an Energy Diagram. 1) Label the axes. The x-axis is labeled as the reaction coordinate, and the y-axis is labeled as energy. 2) Draw a line at the beginning of the graph for the reactants and a line at the end for the products. The products will be higher or lower depending on if the reaction is endothermic or exothermic ...

Label the energy diagram for a two‑step reaction.
Interpreting a Reaction Energy Diagram | Chemistry | Study.com Step 1: Label the reactants and the products and determine their energies Step 2: Identify the activation barrier and its activation energy Step 3: Determine the enthalpy of the... Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes. Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states..
Label the energy diagram for a two‑step reaction.. Energy Diagram For Two Step Reaction Atkinsjewelry The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. check your learning reaction diagrams for a chemical process with and without a catalyst are shown below. both reactions involve a two step mechanism with a rate determining first step. The energy diagram of a two step reaction is shown below ... Drawing the Reaction Energy Diagram of a Catalyzed Reaction For the following simplified equation, label the reaction energy diagram with and without a catalyst. {eq}X + Y \rightarrow Z {/eq} Step 1: Determine the energies of the reactants and the products ... Multistep reaction energy profiles (video) | Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. 6.15: Energy Diagram for a Two-Step Reaction Mechanism A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise Draw structures representing TS1 and TS2 in the reaction above.
Representing endothermic and exothermic processes using energy diagrams ... The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. Question : Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 99% (79 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. Labeling an Energy Diagram Diagram | Quizlet Start studying Labeling an Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools. ... Potential Energy of Reactants for FORWARD reaction and Potential Energy of Products for Reverse Reaction. Students also viewed. CHM 111 Final Exam. 49 terms. olivialogan-us. CHEM 123 SAPLING LEARNING CHAPTER 14. 34 terms. Label the energy diagram for a two-step reaction. Label the energy diagram for a two-step reaction. Two-Step Reaction: This mechanism states that the entire reaction happens in two elementary stages. The overall balanced chemical...
Solved Label the energy diagram for a two-step reaction. - Chegg Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product. Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states.. Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes.
Interpreting a Reaction Energy Diagram | Chemistry | Study.com Step 1: Label the reactants and the products and determine their energies Step 2: Identify the activation barrier and its activation energy Step 3: Determine the enthalpy of the...
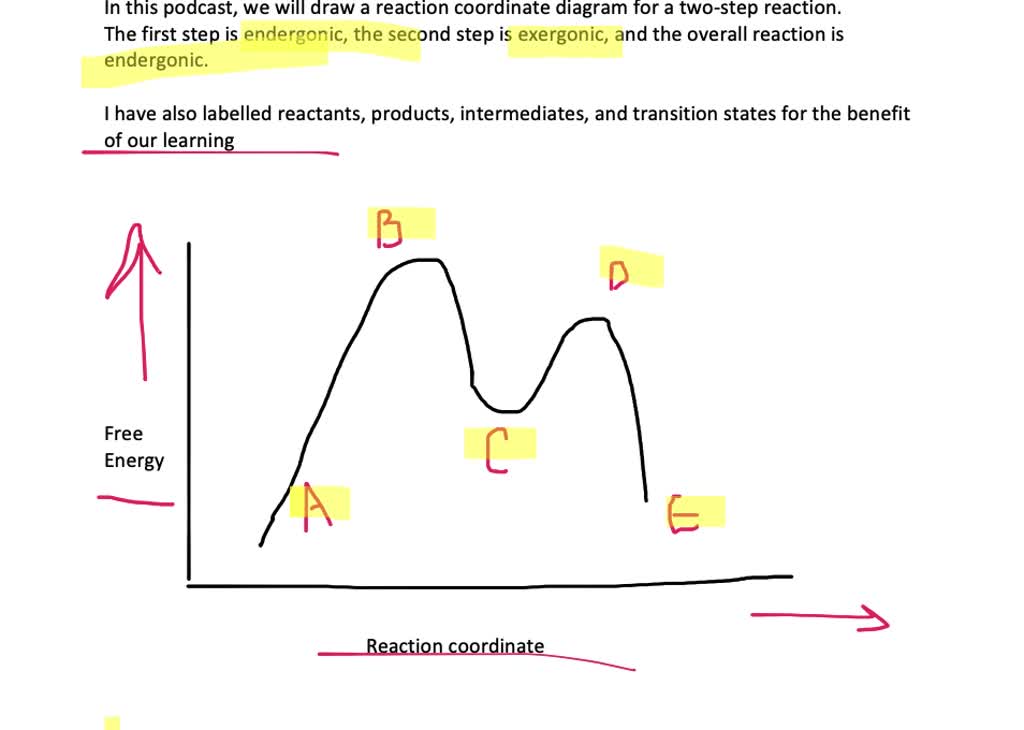
Draw a free energy reaction coordinate diagram for both a, generic endergonic and a generic exergonic reaction (label each)., Make sure to label the x- and y-axes. Now label where reactants and, ...



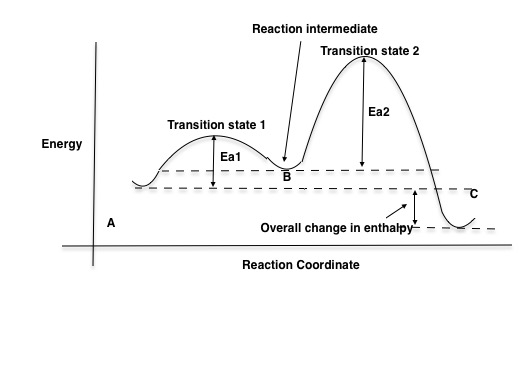



:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)

:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)








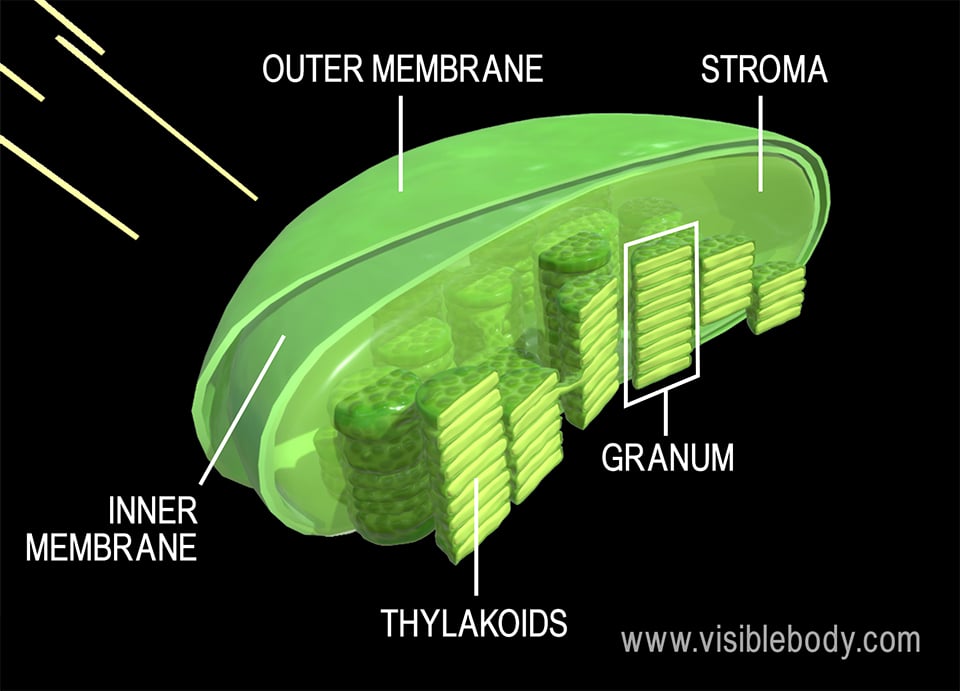
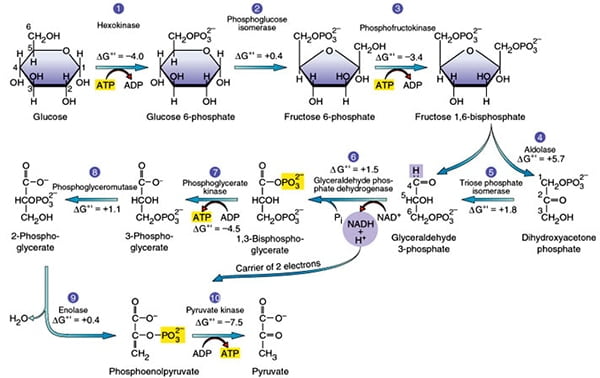



Post a Comment for "42 label the energy diagram for a two‑step reaction."